A kind of synthetic method and application of N heteroatom multi-substituted benzo tetracyclic ketone
A synthesis method and multi-substitution technology, which is applied in the field of synthesis of N-heteroatom multi-substituted benzotetracyclic ketones, can solve problems such as the difficulty of benzocyclobutanone, and achieve high applicability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
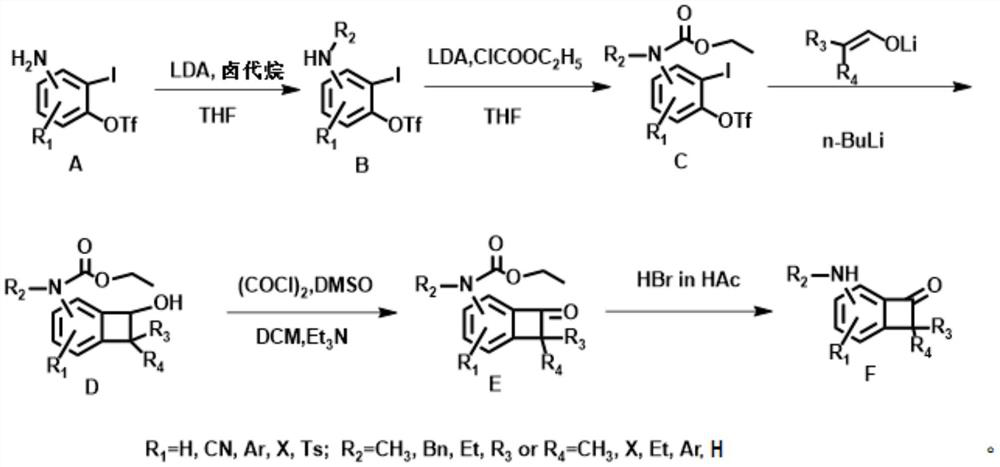
[0032] first step:
[0033]
[0034] Three THF solutions of compound A were prepared, and commercially available LDA (1.08 mmol, 0.54 mL, 2M hexane / THF solution) was added to a solution of compound A (1 mmol) in THF (2 mL) at -78 ° C, and the reactant Stir at this temperature for 20 minutes, add methyl iodide, ethyl iodide and benzyl bromide (63uL) to the three solutions respectively, then continue to stir at room temperature for 1 hour, and wash with NH 4 Quenched with aqueous Cl. Extracted with ethyl acetate, washed with brine, MgSO 4 Drying, concentration and purification by silica gel chromatography afforded compound B1 (R 2 =CH 3 ), B2(R 2 =Et), B3(R 2 =Bn), the yield was 80-91%.
[0035] B1:R f =0.55, (PE:EA=5:1), 1 H NMR (500MHz, Chloroform-d) δ7.27 (td, J = 8.2, 1.2Hz, 1H), 6.73–6.60 (m, 1H), 6.51 (dd, J = 8.4, 1.4Hz, 1H), 2.94 ( d,J=1.3Hz,3H). 13 C NMR (126MHz, Chloroform-d) δ150.83, 150.76, 130.48, 120.15, 117.60, 109.59, 109.58, 109.57, 108.98, 79.59, 3...
Embodiment 2
[0063]
[0064] Concrete experimental operation is with embodiment one, and the product that makes is as follows:
[0065] F4:R f =0.7, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.40(s,1H),7.17(s,1H),3.94(s,2H),3.36(s,3H).
[0066] F5:R f =0.6, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.24(s,1H),7.01(s,1H),3.84(s,2H),3.26(s,3H).
[0067] F6:R f =0.75, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.45(s,1H),7.23(s,1H),3.9(s,2H),3.36(s,3H).
[0068] F7:R f =0.7, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.38(s,1H),7.15(s,1H),3.92(s,2H),3.33(s,3H).
Embodiment 3
[0070]
[0071] Concrete experimental operation is with embodiment one, and the product that makes is as follows:
[0072] F8:R f =0.52, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) 1 H NMR (400MHz, Chloroform-d) δ7.40(d, J=7.26Hz, 1H), 7.14(d, J=7.26Hz, 1H), 6.88(s, 1H), 3.82(s, 2H), 3.09 (s,3H).
[0073] F9:R f =0.57, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) 1 H NMR (400MHz, Chloroform-d) δ7.42 (d, J = 7.26Hz, 1H), 7.16 (d, J = 7.26Hz, 1H), 6.88 (s, 1H), 3.82 (s, 2H), 3.09 (s,3H).
[0074] F10:R f =0.6, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.32(d, J=4.8Hz, 4H), 7.30–7.26(m, 1H), 7.21(t, J=4.8Hz, 1H), 6.7(d, J= 7.0Hz, 1H), 6.45(d, J=8.4Hz, 1H), 4.64(s, 2H), 3.80(s, 2H).
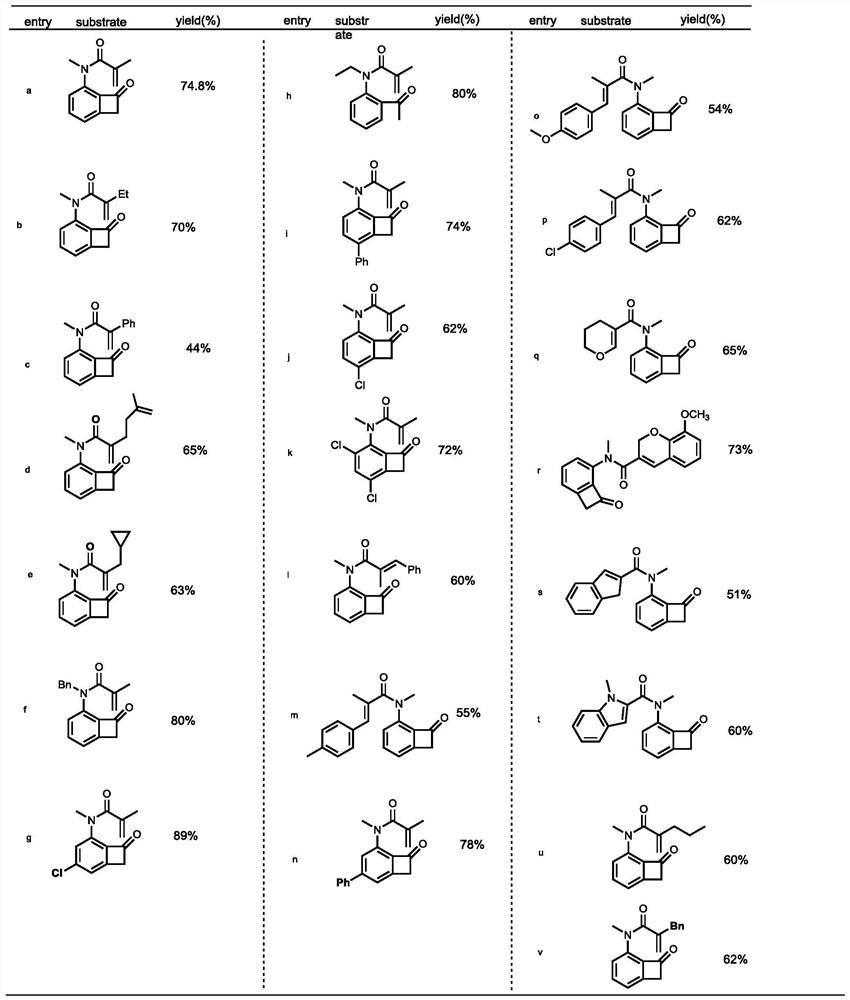
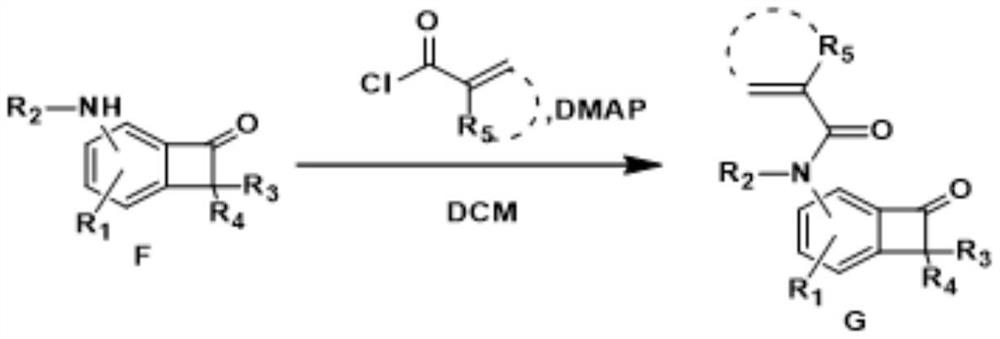
[0075] Part II: Application of N heteroatom multi-substituted benzotetracyclic ketones
[0076] The first step, the preparation of acid chloride:
[0077] figure 1 Among them, the preparation process of the required acid chlorides for Gb, Gd, Ge, Gq, Gt is consistent, as follows:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com