Dual-cavity nasal spray containing oxymetazoline hydrochloride and used for treating rhinitis
A technology of oxymetazoline hydrochloride and oxymetazoline acid, which is applied in the field of medicine, can solve problems such as complex compatibility and limited application, achieve strong adhesion, restore normal function, and improve nasal cavity comfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
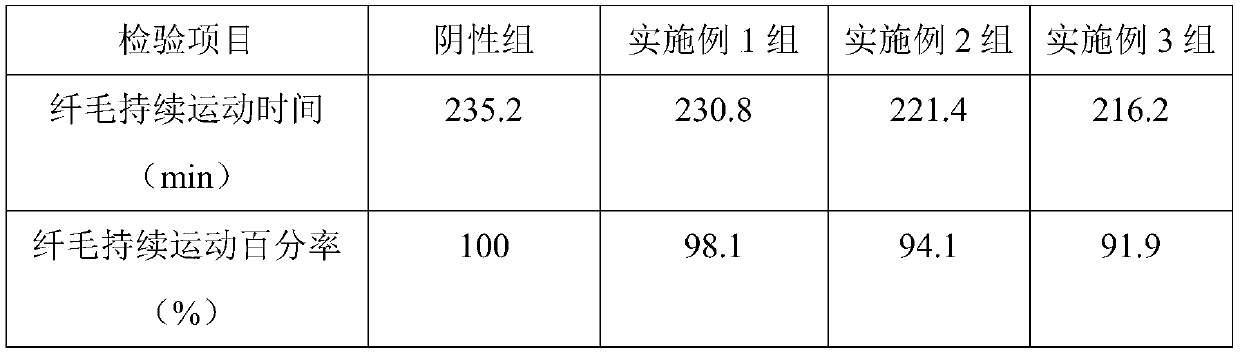
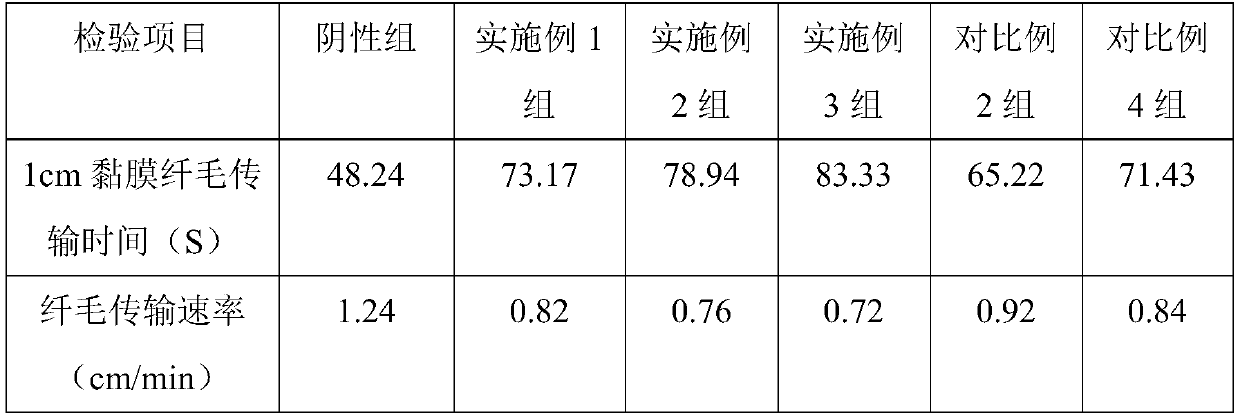
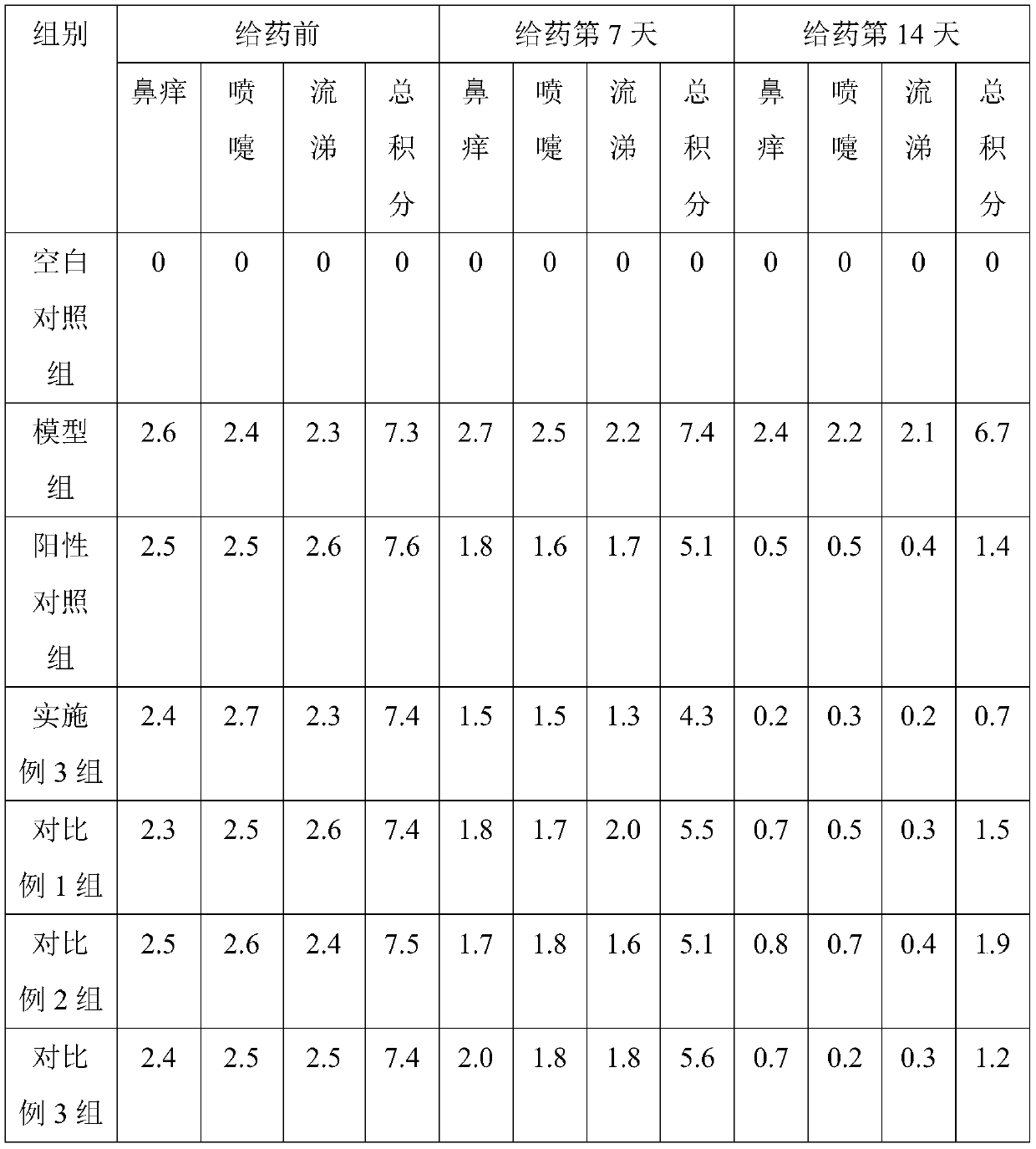
[0039] Embodiment 1, a kind of two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis
[0040] The two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis is provided with two conjoined spray chambers, and the aqueous solution containing oxymetazoline hydrochloride and the Glycoside emulsion.
[0041] The aqueous solution containing oxymetazoline hydrochloride comprises the following components and mass percentages thereof: 0.01% oxymetazoline hydrochloride, 0.5% sodium chloride, 0.4% functional auxiliary agent, and 99.09% purified water; the functional auxiliary agent consists of Chitooligosaccharide and sodium alginate are composed in a mass ratio of 6:3; the average molecular weight of the chitooligosaccharide is 1000.
[0042] The preparation method of the aqueous solution containing oxymetazoline hydrochloride is:
[0043] Add oxymetazoline hydrochloride to purified water, stir until completely d...
Embodiment 2
[0051] Embodiment 2, a kind of two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis
[0052] The two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis is provided with two conjoined spray chambers, and the aqueous solution containing oxymetazoline hydrochloride and the Glycoside emulsion.
[0053] The aqueous solution containing oxymetazoline hydrochloride comprises the following components and mass percent thereof: 0.02% oxymetazoline hydrochloride, 1% sodium chloride, 0.6% functional auxiliary agent, and 98.38% purified water; the functional auxiliary agent consists of Oligochitosan and sodium alginate are composed at a mass ratio of 8:1; the average molecular weight of the oligochitosan is 2500.
[0054] The preparation method of the aqueous solution containing oxymetazoline hydrochloride is:
[0055] Add oxymetazoline hydrochloride to purified water, stir until completely dissolved, add functi...
Embodiment 3
[0063] Embodiment 3, a kind of two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis
[0064] The two-chamber nasal spray containing oxymetazoline hydrochloride for the treatment of rhinitis is provided with two conjoined spray chambers, and the aqueous solution containing oxymetazoline hydrochloride and the Glycoside emulsion.
[0065] The aqueous solution containing oxymetazoline hydrochloride comprises the following components and mass percentages thereof: 0.012% oxymetazoline hydrochloride, 0.7% sodium chloride, 0.5% functional auxiliary agent, and 98.788% purified water; the functional auxiliary agent consists of Chitooligosaccharide and sodium alginate are composed in a mass ratio of 7:2; the average molecular weight of the chitooligosaccharide is 1500.
[0066] The preparation method of the aqueous solution containing oxymetazoline hydrochloride is:
[0067] Add oxymetazoline hydrochloride to purified water, stir until completely...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com