Placental implantable disease markers
A placenta accreta and disease technology, applied in the field of placenta accreta disease markers, can solve problems such as no further application, unclear clinical significance, and high technical requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Materials and equipment
[0040] Kit: MagMAX mirVana Total RNA Isolation Kit
[0041] Instrument model: 7900HT real-time fluorescence quantitative instrument of ABI company
[0042] Chip: miRCURY-Ready-to-Use PCR-Human-panel-I+II-V4.M
[0043] 2. Collection of peripheral blood samples
[0044] After obtaining the informed consent of the pregnant women, the non-anticoagulated peripheral blood serum of suspected cases and the non-anticoagulated peripheral blood serum of normal delivery pregnant women were collected before delivery. After coagulation at room temperature for 30-45 minutes, centrifuge at 4°C at 2000g for 15 minutes. minutes to separate the serum and cells, and then transfer the separated serum to a cryopreservation tube. Store in a -80°C ultra-low temperature freezer. After the samples were collected, 200 μl of serum was melted at 4°C, and centrifuged repeatedly at 2000 g for 10 minutes to completely remove platelets and other precipitates.
[0045] ...
Embodiment 2
[0055] 1. Materials and equipment
[0056] Kit: MagMAX mirVana Total RNA Isolation Kit
[0057] Instrument model: Q-PCR instrument of ABI company
[0058] 2. Collection of peripheral blood samples
[0059] After obtaining the informed consent of the pregnant women, the non-anticoagulated peripheral blood serum of suspected cases and the non-anticoagulated peripheral blood serum of normal delivery pregnant women were collected before delivery. After coagulation at room temperature for 30-45 minutes, centrifuge at 4°C at 2000g for 15 minutes. minutes to separate the serum and cells, and then transfer the separated serum to a cryopreservation tube. Store in a -80°C ultra-low temperature freezer. After the samples were collected, 200 μl of serum was melted at 4°C, and centrifuged repeatedly at 2000 g for 10 minutes to completely remove platelets and other precipitates.
[0060] 3. Extraction of serum total RNA
[0061] According to the method provided by the manufacturer, tot...
Embodiment 3
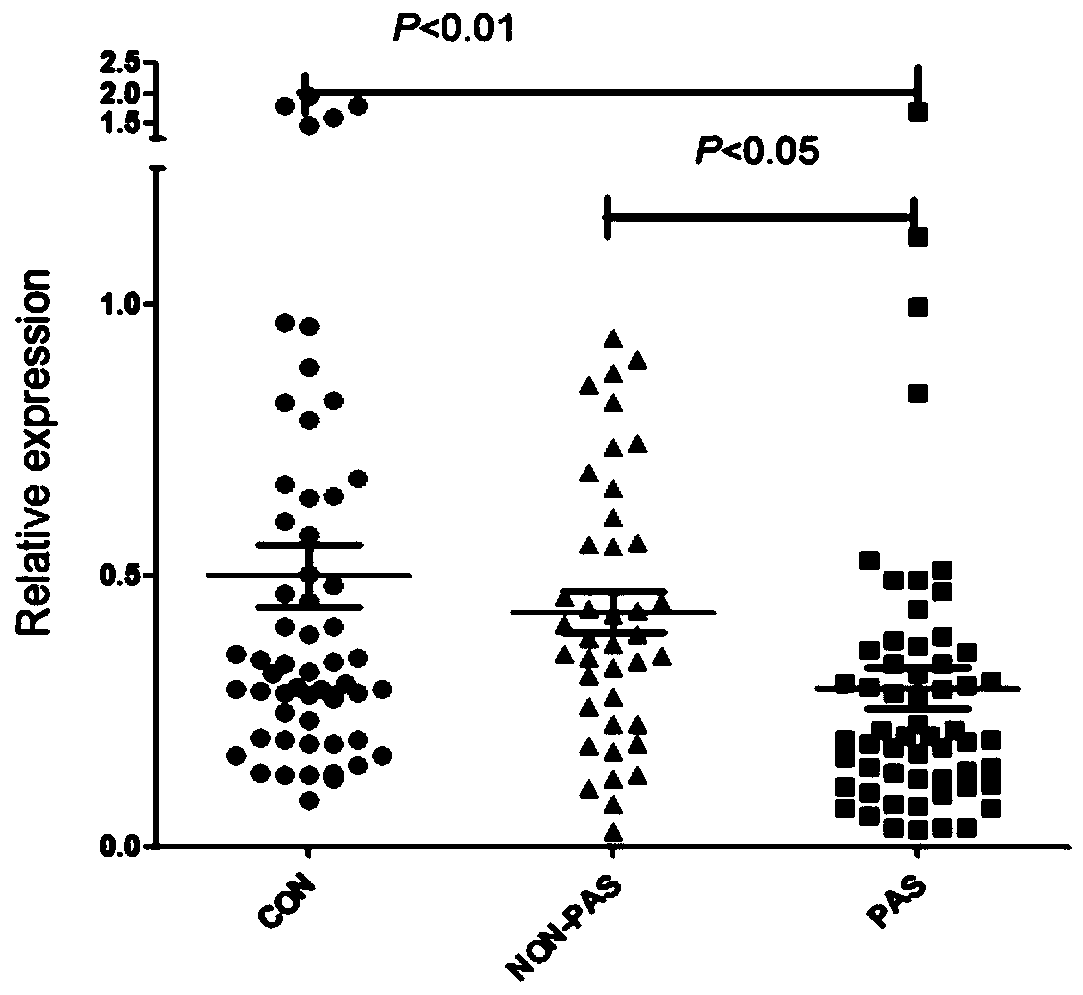
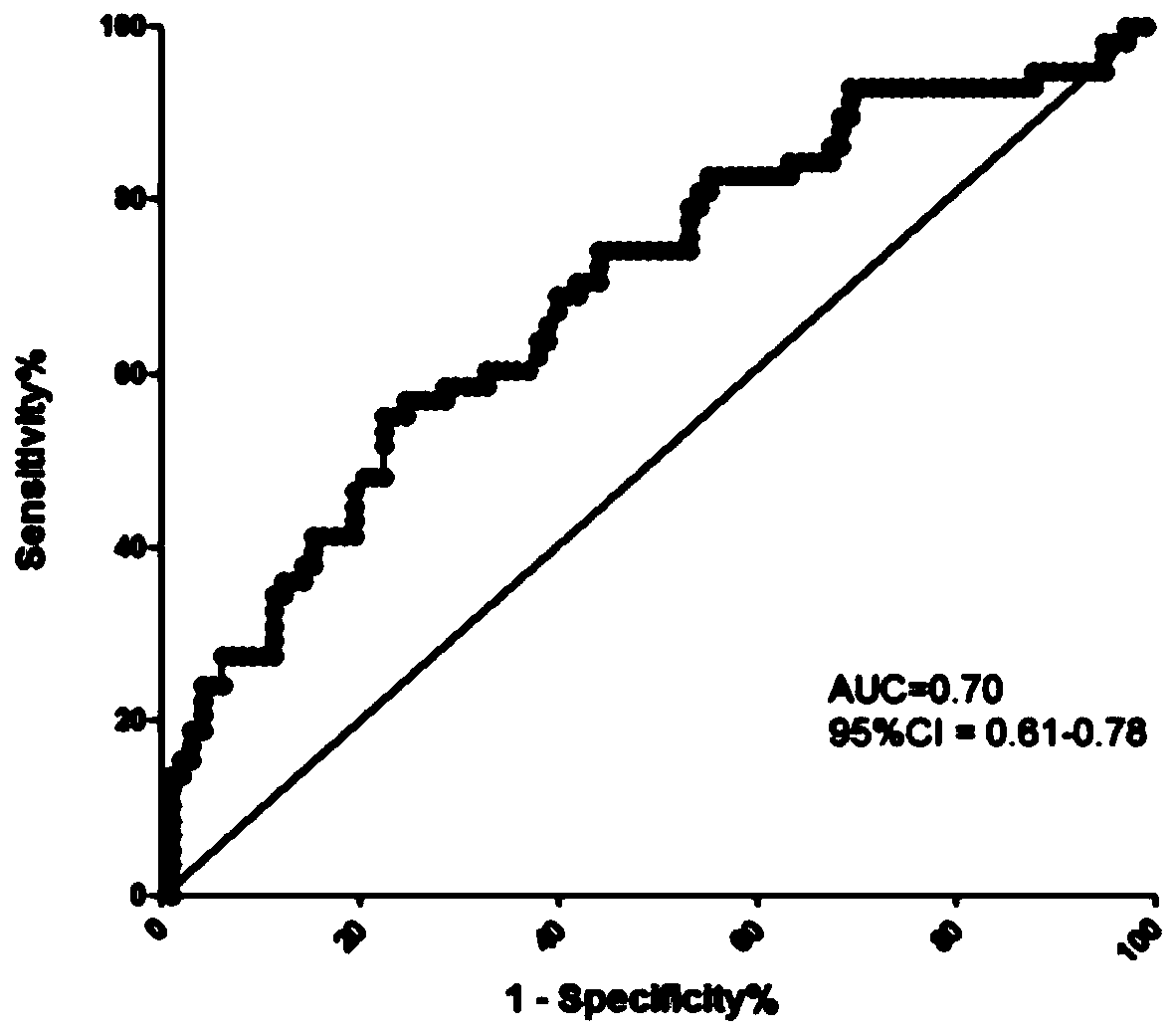
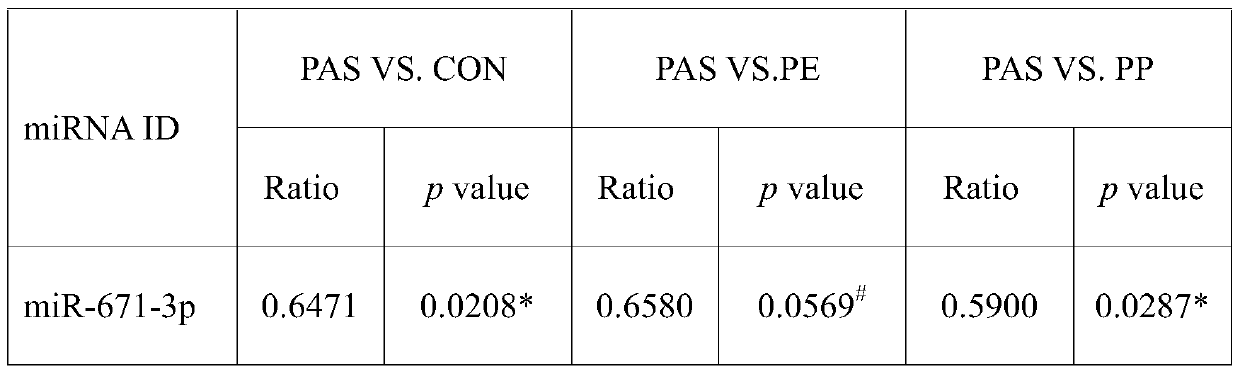
[0074]Using the same method as in Example 2, the third group of people was tested, and the expression of miR-671-3p was performed on the non-anticoagulated peripheral blood serum collected before delivery from pregnant women with bleeding tendency and normal pregnant women. volume detection. The specific diagnostic results are obtained from the pathological tissue analysis of pregnant women after delivery. The true positive results are confirmed cases of placenta accreta, and the false positive results include cases of placenta previa and preeclampsia. The final number is 20 cases of placenta accreta, 20 cases of placenta previa, 20 cases of preeclampsia, and 20 normal controls.
[0075] Table 2 The multiple of change and P value among different groups in the third batch of population
[0076]
[0077] Ratio: The expression ratio between different groups. PAS: placenta accreta; CON: normal delivery; PP: placenta previa; PE: preeclampsia; *: p<0.05 by t-test between two gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com