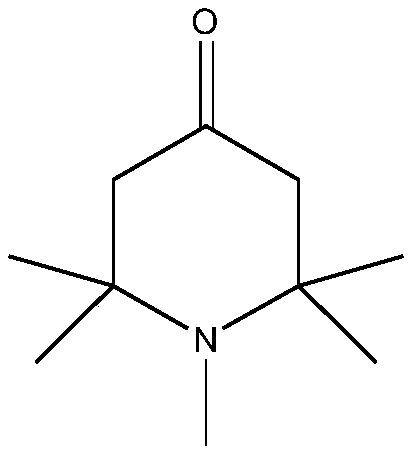
Preparation method of 1, 2, 2, 6, 6-pentamethyl-4-piperidone
A technology of tetramethylpiperidone and piperidone, applied in the field of chemical synthesis, can solve the problems of difficult industrialization and high cost, and achieve the effect of increasing yield and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
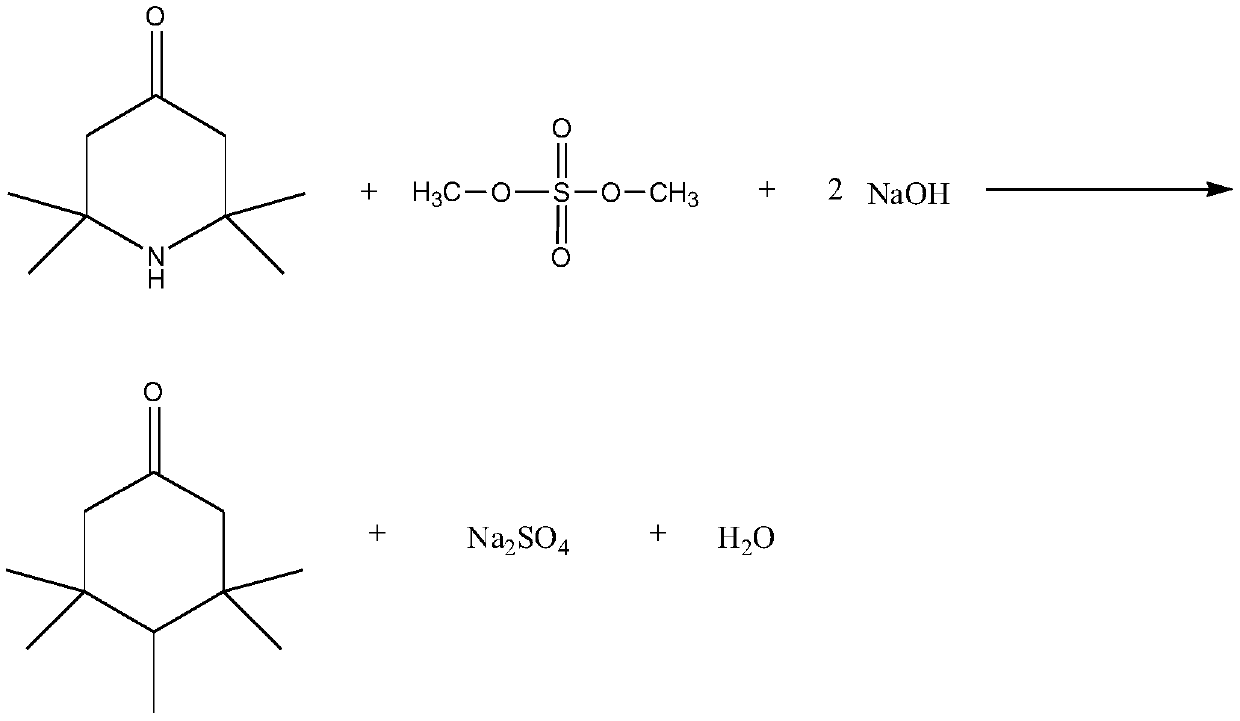
[0026] In a 500ml four-neck flask equipped with a thermometer, add 155g of 2,2,6,6-tetramethylpiperidone, 300g of acetone, and 100g of sodium hydroxide, install a condenser, and slowly add 150g of dimethyl sulfate dropwise, And control temperature is 35 ℃. The dropwise addition was completed within 2 hours, and then the reaction was continued at this temperature for 5 hours. After cooling and filtering, the filtrate was concentrated and rectified to obtain the final product 1,2,2,6,6-pentamethyl-4-piperidone with a yield of 95%.
Embodiment 2
[0028] In a 500ml four-neck flask equipped with a thermometer, add 50g of 2,2,6,6-tetramethylpiperidone, 80g of acetonitrile, and 80g of sodium carbonate, install a condenser, slowly add 60g of dimethyl sulfate dropwise, and The control temperature is 45°C. The dropwise addition was completed within half an hour, and then the reaction was continued at this temperature for 3 hours. After cooling and filtering, the filtrate was concentrated and rectified to obtain the final product 1,2,2,6,6-pentamethyl-4-piperidone with a yield of 96%.
Embodiment 3
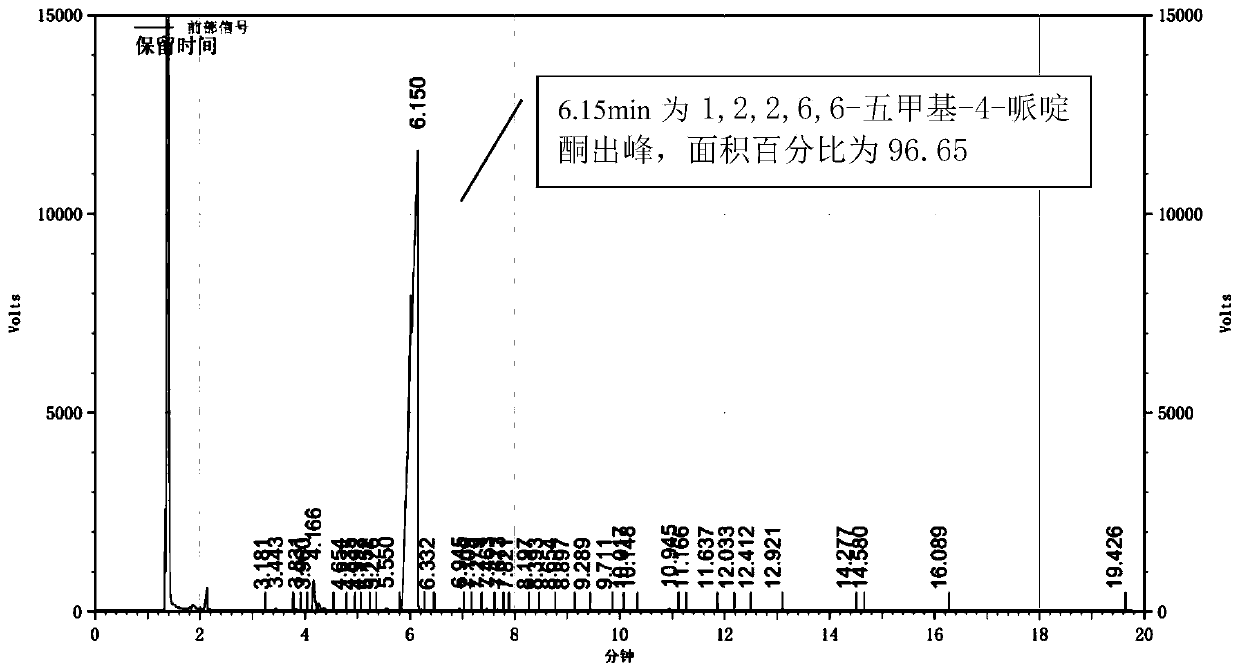
[0030] In a 500ml four-necked flask equipped with a thermometer, add 100g of 2,2,6,6-tetramethylpiperidone, 210g of DMF, and 200g of potassium carbonate, install a condenser, slowly add 120g of dimethyl sulfate dropwise, and control The temperature is 50°C. The dropwise addition was completed within 2 hours, and then the reaction was continued at this temperature for 4 hours. After cooling and filtering, the filtrate was concentrated and rectified to obtain the final product 1,2,2,6,6-pentamethyl-4-piperidone with a yield of 96.6%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap