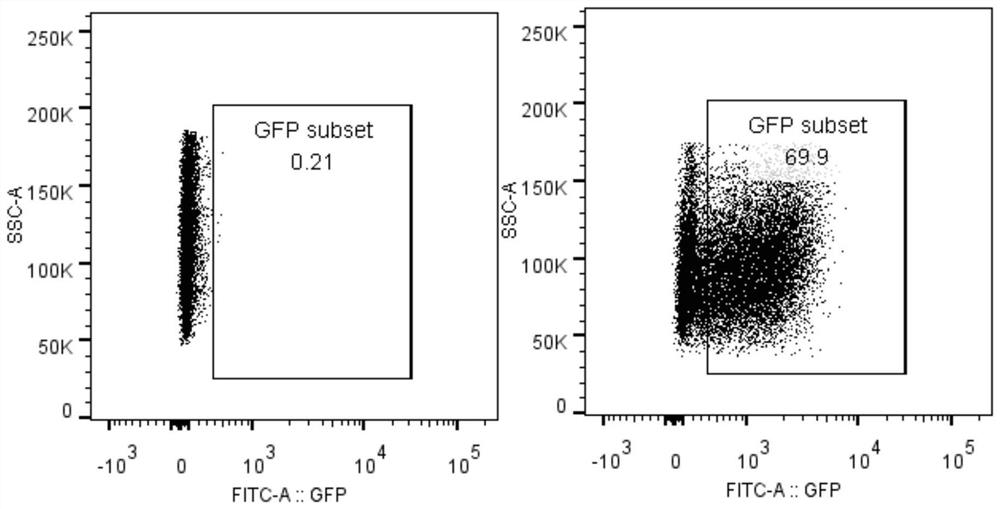
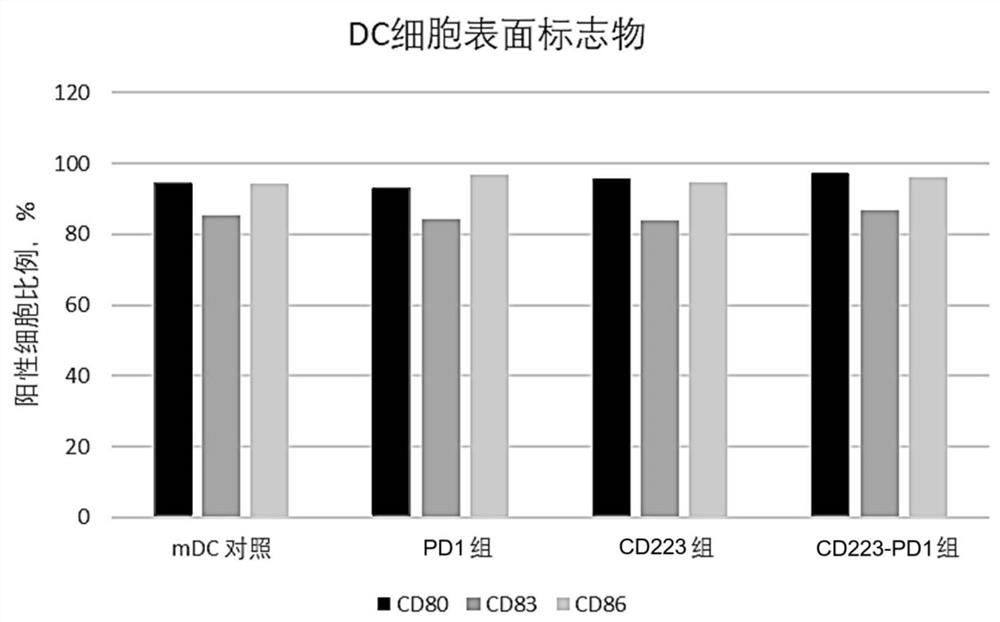
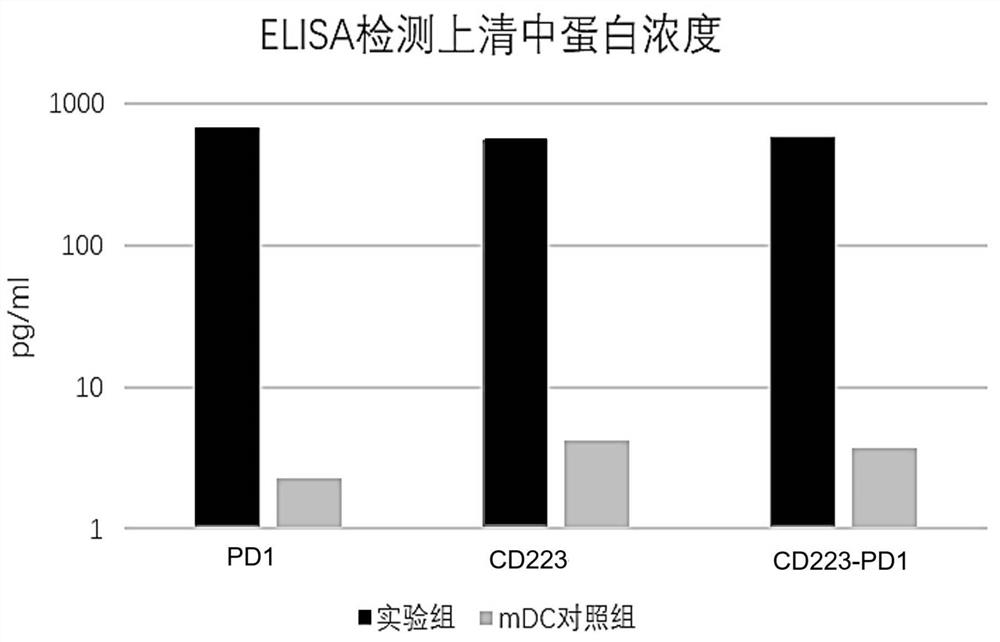
Fusion protein, encoding nucleic acid and cell and use thereof
A fusion protein and cell technology, which is applied in the field of immunosuppressants to relieve immunosuppression, can solve the problems of slow disease progression and achieve the effect of enhancing anti-tumor function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The method for preparing the cells of the present invention is not particularly limited. In an exemplary preparation method, the preparation method of the cell of the present invention comprises the following steps:
[0044] (1) Construct a nucleic acid capable of producing a fusion protein;
[0045] (2) Peripheral blood mononuclear cells are isolated from venous blood and induced to differentiate to obtain antigen-presenting cells; and
[0046] (3) introducing the nucleic acid of step (1) into the antigen-presenting cells of step (2), and culturing the antigen-presenting cells under conditions suitable for expression of the nucleic acid.
[0047] In certain embodiments, the methods of the invention comprise preparing a plasmid comprising DNA encoding the corresponding. Afterwards, the in vitro transcription process is carried out. Firstly, the plasmid is linearized with a restriction endonuclease, and the ribonucleic acid molecule is prepared by in vitro transcriptio...
Embodiment 1
[0056] This preparation example is a method for preparing an exemplary fusion protein.
[0057] 1. Preparation of DNA and mRNA Constructs
[0058] The gene used to generate an exemplary fusion protein of the present invention is constructed, and its sequence is shown in SEQ ID No. 5. In addition, genes used to produce soluble immunoglobulin CD223 and soluble programmed death receptor 1 as controls were further constructed, and their sequences are shown in SEQ ID No. 6 and 7, respectively. In addition, the antigen GPC3 used in subsequent experiments is encoded by the sequence shown in SEQ ID No. 8. The sequence information is shown in Table 1 below.
[0059] Table-1 DNA sequence list
[0060]
[0061] 2. In vitro transcription
[0062] Firstly, the prepared corresponding DNA plasmid was linearized using restriction endonuclease, and the linearized plasmid was used as a template, and T7 RNA polymerase was used to transcribe in vitro to prepare mRNA. The prepared mRNA was...
Embodiment 2
[0075] The experiment was performed in the same manner as in Example 1 except that the antigen in Example 1 was changed to AFP. The results are shown in the table below.
[0076] T cell control group mDC control AFP group AFP + PD-1 group AFP + CD223 group AFP+CD223-PD-1 group CD8 IFN-r+ 0.049 0.026 0.029 0.064 0.248 0.46 CD8 TNF-a+ 0.049 0 0 0.021 0.262 0.236 CD8 TNF-a+, IFN-r+ 0.049 0 0 0 0.18 0.15 CD4 IFN-r+ 0.3 0.027 0 0.24 0.165 0.64 CD4 TNF-a+ 0.201 0.28 0.27 0.35 0.37 1 CD4 TNF-a+, IFN-r+ 0.15 0 0 0.13 0.14 0.14
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com