A method for preparing 3-mercapto-5-methyl-1,2,4-triazole from triazine ring
A triazine ring and triazole technology, applied in the field of organic chemical synthesis, can solve the problems of high process complexity and low conversion rate, and achieve the effects of simple process flow, less environmental pollution, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing 3-mercapto-5-methyl-1,2,4-triazole from a triazine ring, comprising the following steps in turn:
[0028] (1) Take 25.2g of triazine ring, add 352.8g of tap water, start stirring to accelerate the dissolution.
[0029] (2) Add 16.5g of 30% hydrochloric acid to adjust the pH of the solution to 2.5.
[0030] (3) Heat up to 72°C and start reflux for 3 hours.
[0031] (4) Suction filtration at 32°C.
[0032] (5) Distillation under reduced pressure The vacuum degree is -0.065 MPa, the temperature is controlled at 53°C, distill until a white solid appears, and the distillation time is 2.1h.
[0033] (6) Cool down to 18°C and filter with suction to obtain a wet product.
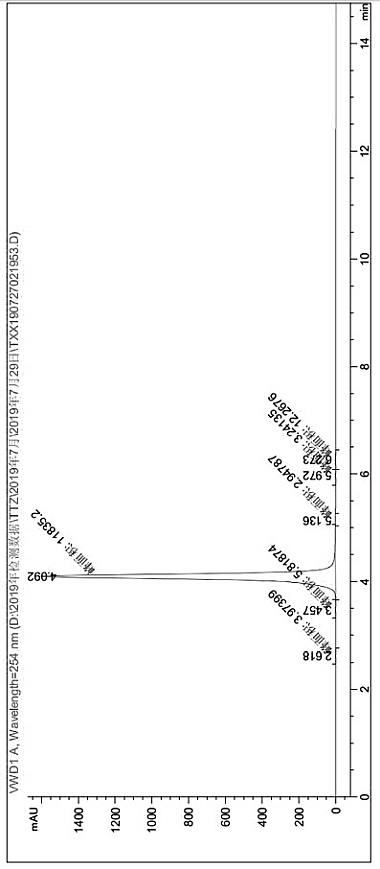
[0034] (7) The product quality after drying was 15.2g, and the product obtained by liquid chromatography was 3-mercapto-5-methyl-1,2,4-triazole, the product purity was 99.5115%, and the conversion rate was 83 %.
Embodiment 2
[0036] A method for preparing 3-mercapto-5-methyl-1,2,4-triazole from a triazine ring, comprising the following steps in turn:
[0037] (1) Take 19.3g of triazine ring, add 270.2g of tap water, start stirring to accelerate the dissolution.
[0038] (2) Add 11.1g of 30% hydrochloric acid to adjust the pH of the solution to 2.5.
[0039] (3) Heat up to 72°C and start reflux for 3 hours.
[0040] (4) Suction filtration at 30°C.
[0041] (5) Distillation under reduced pressure The vacuum degree is -0.068 MPa, the temperature is controlled at 51°C, distill until a white solid appears, and the distillation time is 2h.
[0042] (6) Cool down to 16°C and filter with suction to obtain a wet product.
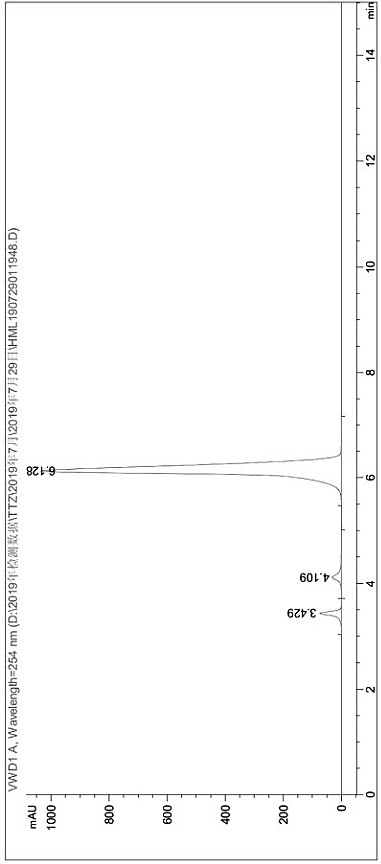
[0043] (7) The product quality after drying was 11.87g, and the product obtained by liquid chromatography was 3-mercapto-5-methyl-1,2,4-triazole, the product purity was 99.5431%, and the conversion rate was 85 %.
[0044] pass figure 1 and figure 2 Two liquid phase detection spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com