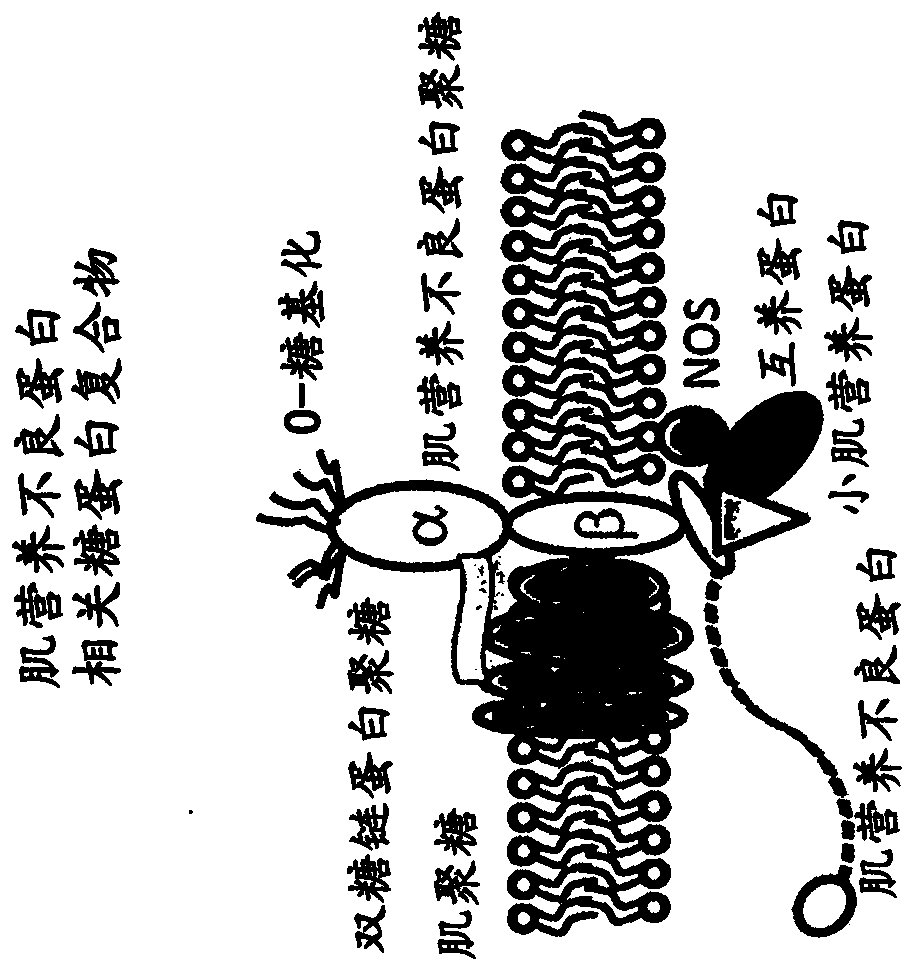
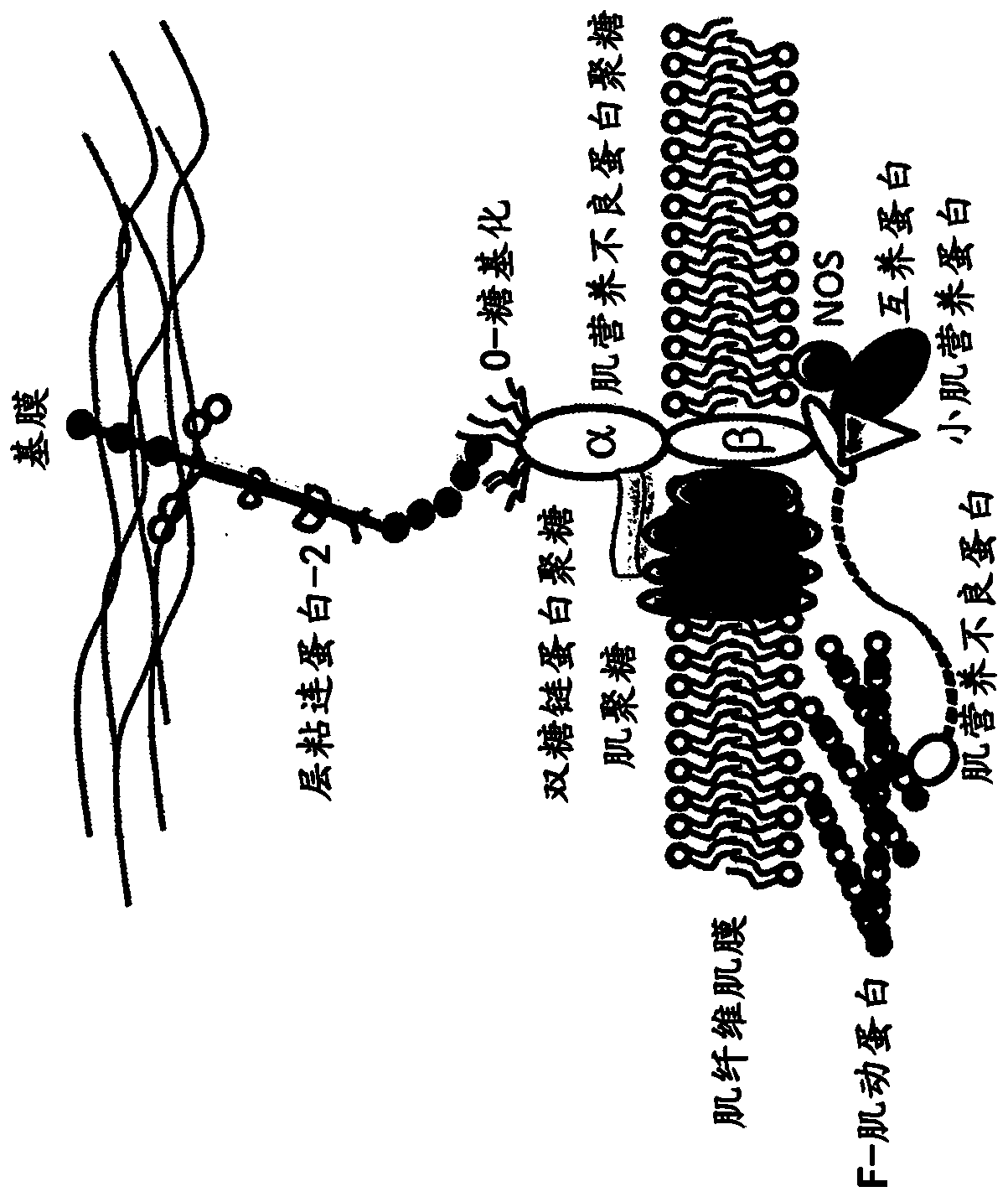
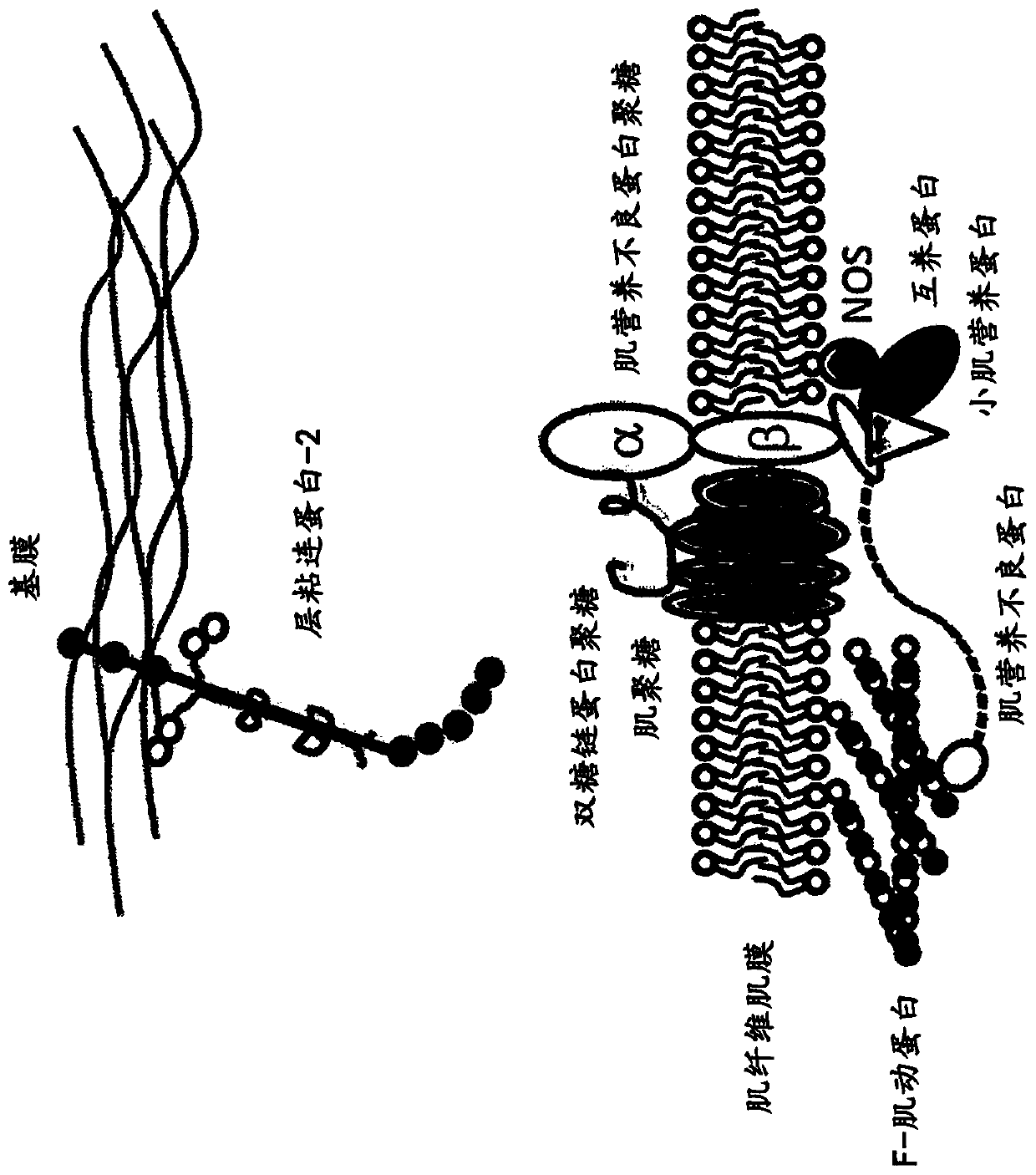
Multispecific binding molecules having specificity to dystroglycan and laminin-2
A muscular dystrophy, multispecific technology, applied in the field of multispecific binding molecules, which can solve problems such as impracticality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0494] Example 1: Identification of anti-β-DG ECD, anti-LG-5 and anti-LG-4 / 5 antibodies.
[0495] method
[0496] protein expression
[0497] For expression of the murine β-DG extracellular domain (mβ-DG ECD), a construct containing an E. coli codon-optimized cassette encoding the N-terminal maltose-binding protein, TEV cleavage site, mβ-DG ( UniProt Q62165, amino acids 652-746) and a C-terminal HPC4 tag, with pET22b as the parental vector backbone. The constructs were transformed into chemically competent Origami B(DE3)pLysS cells (Novagen). Expression was performed at 37°C and IPTG induction was performed at OD = 0.6. Cells were pelleted and resuspended in lysis buffer containing EDTA-free protease inhibitors (Roche) and lysed by sonication. mβ-DG-HPC4 was purified from clarified cell lysates by treating the cell lysates on an amylose resin column (NewEngland Biolabs), cleaving the maltose-binding protein with Turbo TEV protease (EtonBiosciences), The digest was proce...
Embodiment 2
[0508] Example 2: Generation of hybridomas, monoclonal and chimeric antibodies targeting β-DG, LG-5 and LG-4 / 5.
[0509] method
[0510] Cell line production
[0511] By codon-optimizing constructs containing the extracellular and endogenous transmembrane domains of the N-terminal myc tag and β-DG (mouse UniProtQ62165, amino acids 652-893; human UniProt Q14118, amino acids 654-895), the Stable cell lines expressing human or murine β-DG on the surface. Adherent human embryonic kidney cells (HEK) and adherent Chinese hamster ovary cells (CHO-K1 ) were transfected using lipofectamine (ThermoFisher), and cells were selected with Geneticin (Gibco). Surviving cells were serially diluted to achieve uniclonal capacity and surface expression of β-DG was confirmed by anti-myc flow cytometry.
[0512] Construction of Tfr1 transmembrane domain containing N-terminal myc tag, Gly / Ser linker, LG-5 (mouse UniProt Q60675, amino acids 2932-3118; human UniProt P24043, amino acids 2936-3122)...
Embodiment 3
[0546] Example 3: Generation of bispecific antibodies recognizing β-DG and the LG-4 / 5 domain of the laminin-2α subunit
[0547] method
[0548] Generation of tetravalent bispecific tandem Ig (TBTI) antibodies
[0549] The VH and VL sequences obtained from the resulting hybridoma cells were codon optimized and synthesized for mammalian expression (Genscript). To generate a construct expressing the light chain, a VL sequence specific for β-DG, (G4S) 2 The linker, a VL sequence specific to LG-4 / 5, and human κ chain (Genbank Q502W4) or mouse κ chain (Genbank BAB33404) were fused together and cloned into the transient episomal expression vector pXL, which was derived from An analogue of the pTT vector described by Durocher et al. (Nucl. Acids Res. 2002, 30(2), E9). To generate a construct expressing the heavy chain, a VH sequence specific for β-DG, (G4S) 2 A linker, a VH sequence specific for LG-4 / 5, and human IgG1 (Genbank Q569F4) or mouse IgG1 (GenBank AAA75163.1) fused toget...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com