A kind of drug-loaded synovial fluid additive and its preparation method and application
A joint synovial fluid and loaded technology, which is applied in the direction of drug combination, pharmaceutical formula, antipyretic, etc., can solve the problems that the synthesis preparation technology has not yet been established, and achieve the benefits of large-scale production, lower surface energy, and prolong lubrication time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of Tris-HCl buffered saline solution (TBS solution) with pH=8.5: Accurately weigh 0.12114 g of Tris-HCl solid, add 20 mL of ultrapure water to dissolve, and adjust the pH to 8.5 with concentrated HCl.
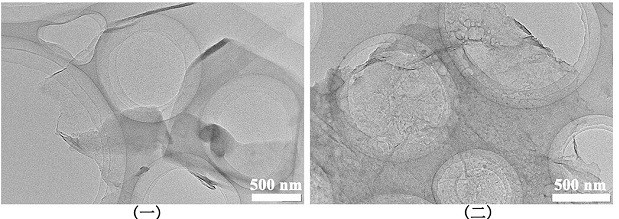
[0040] (2) Accurately weigh 1 g of HA, dissolve 50 mg of FGO in 100 mL of TBS solution with pH=8.5, and sonicate for 40 min; add 500 mg of DA-HCl, and use HCl to adjust the pH to 8.5; the oxygen content of FGO is 45%, and the fluorine content is 55%.
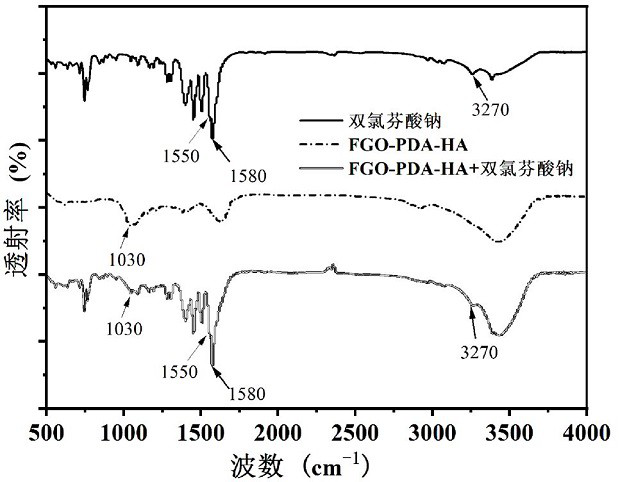
[0041] (3) After mixing evenly, stir, protect from light, and heat at 60 °C for 24 h under nitrogen protection. After the reaction, the above solution was put into a dialysis bag and dialyzed in deionized water for 1 day. It was transferred into a beaker, sealed with filter paper, and freeze-dried to obtain a dopamine, hyaluronic acid-modified fluorinated graphene sample (FGO-PDA-HA).
Embodiment 2
[0043] (1) Prepare Tris-HCl buffered saline solution (TBS solution) with pH=8.0: Accurately weigh the Tris-HCl solid, add ultrapure water to dissolve, and adjust the pH to 8.0 with concentrated HCl.
[0044](2) Accurately weigh 2 g of HA, dissolve 100 mg of FGO in 200 mL of TBS solution with pH=8.0, and sonicate for 40 min; add 500 mg of DA, and use HCl to adjust the pH to 8.0; the oxygen content of FGO is 95%, The fluorine content is 5%.
[0045] (3) After mixing evenly, stir, protect from light, and heat at 80 °C for 7 h under nitrogen protection. After the reaction, the above solution was put into a dialysis bag and dialyzed in deionized water for 1 day. It was transferred into a beaker, sealed with filter paper, and freeze-dried to obtain a dopamine, hyaluronic acid-modified fluorinated graphene sample (FGO-PDA-HA).
Embodiment 3
[0047] (1) Prepare Tris-HCl buffered saline solution (TBS solution) with pH=10: Accurately weigh the Tris-HCl solid, add ultrapure water to dissolve, and adjust the pH to 10 with concentrated HCl.
[0048] (2) Accurately weigh 1 g of HA, dissolve 100 mg of FGO in 200 mL of TBS solution with pH=10, and sonicate for 40 min; add 10 mg of DA-HCl, and use HCl to adjust the pH to 9.5; the oxygen content of FGO is 5%, and the fluorine content is 95%.
[0049] (3) After mixing evenly, stir, protect from light, and heat at 25 °C for 36 h under helium protection. After the reaction, the above solution was put into a dialysis bag and dialyzed in deionized water for 1 day. It was transferred into a beaker, sealed with filter paper, and freeze-dried to obtain a dopamine, hyaluronic acid-modified fluorinated graphene sample (FGO-PDA-HA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| friction coefficient | aaaaa | aaaaa |
| friction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com