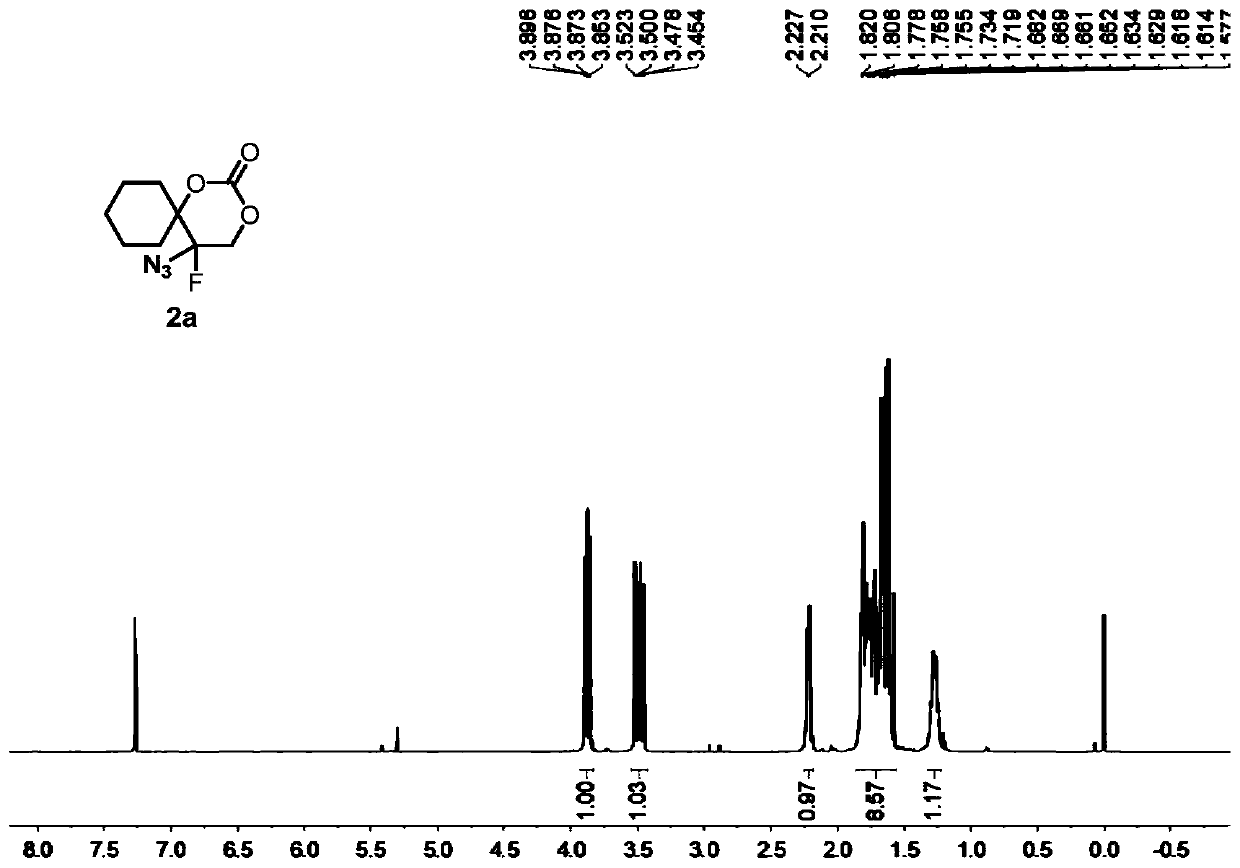
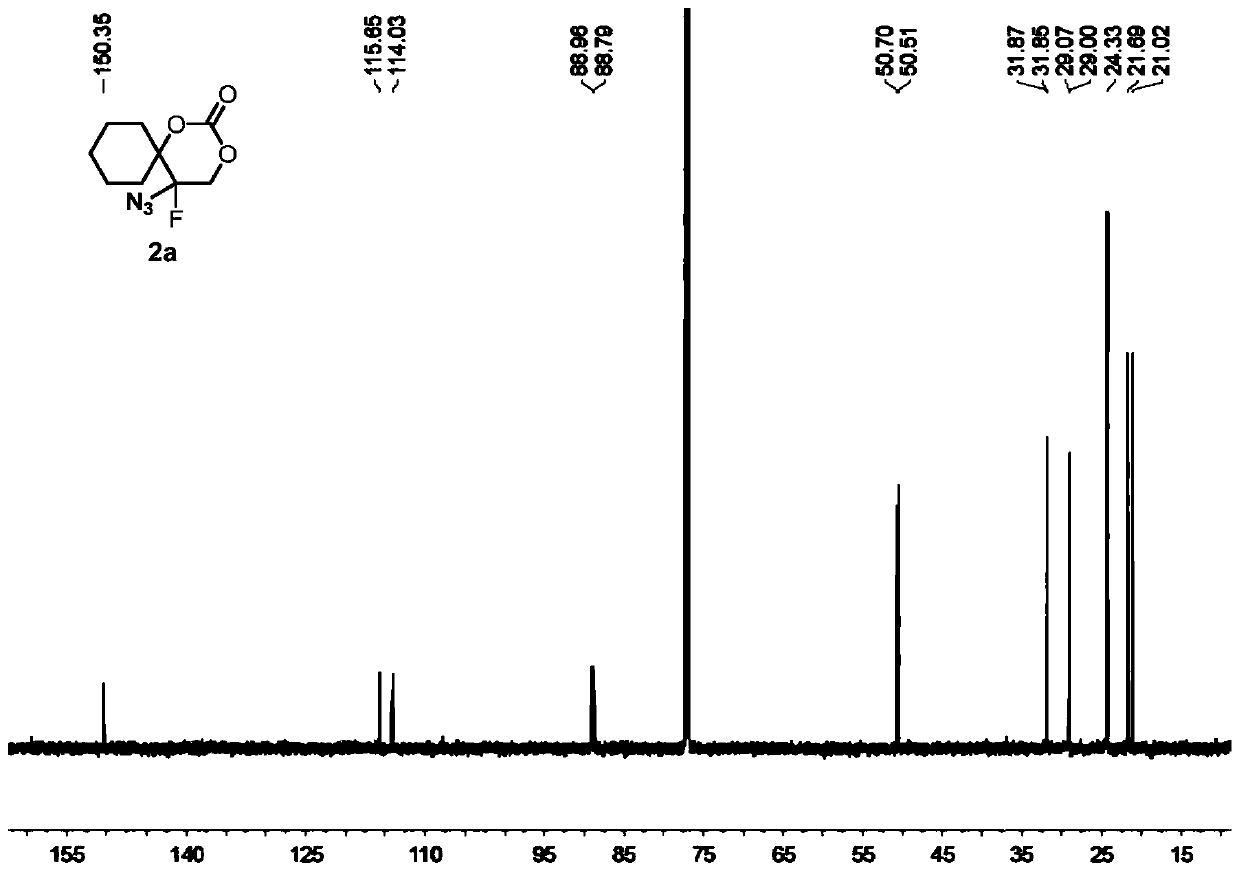
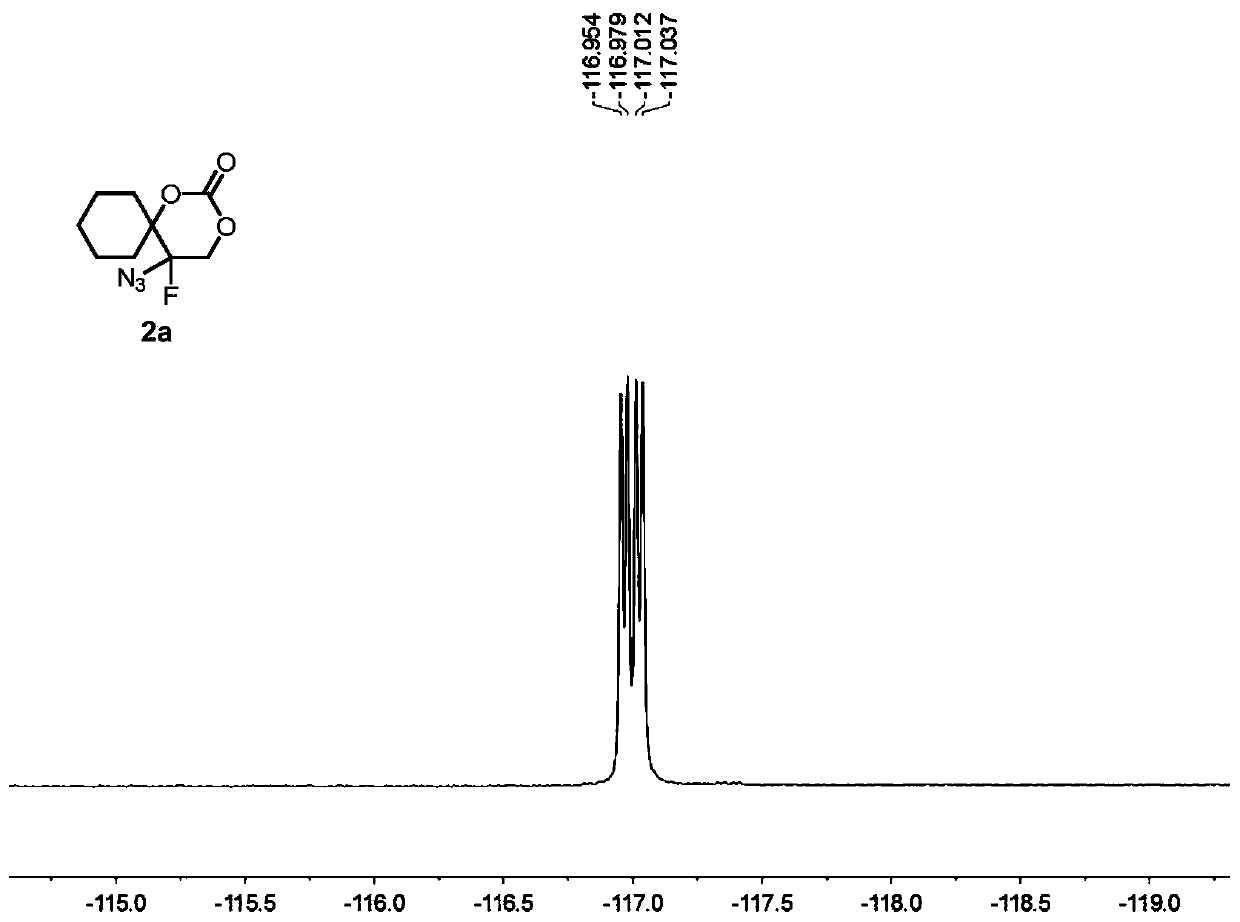
5-azide-5-fluoro-1,3-dioxane-2-one derivative and preparation method thereof
A technology of dioxocyclyl derivatives, applied in the field of preparation of vinyl azide compounds, to achieve the effects of good functional group tolerance, moderate yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Compound 1a (i.e. 1-(1-azidoethenyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PIDA (0.3995g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, Py·HF (0.4956 g, 5 mmol) was added under stirring and stirring was continued until the complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). At -78° C., the suspended silica ( 15 g per 1 mmol of substrate), the resulting heterogeneous mixture was then transferred to a suspension in ethyl acetate, and the suspension was allowed to warm to room temperature. The resulting suspension was then filtered and extracted with dichloromethane (3×15 mL) for 3 times, washed 3 times with brine (3×40mL).Finally, the combined organic layer was washed with Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =10:3), the colorless oily product 2a was obtained with a yield of 96%.
Embodiment 2
[0086]Compound 1a (i.e. 1-(1-azidovinyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PhIO (0.3301g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, Add Et with stirring 3 N. HF (0.4956 g, 5 mmol), continued stirring until complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). The silica (15 g per 1 mmol of substrate) was vigorously stirred to suspend the suspension at -78°C, then the resulting heterogeneous mixture was transferred to a suspension in ethyl acetate, which was allowed to warm to room temperature. The resulting suspension was then filtered, extracted three times with dichloromethane (3 x 15 mL) and washed once with brine (3 x 40 mL). Finally, the combined organic layers were subjected to Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =5:1), the colorless oily product 2a was obtained with a yield of 71%.
Embodiment 3
[0088] Compound 1a (i.e. 1-(1-azidoethenyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PIFA (0.6451g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, AgF ((0.4956 g, 5 mmol) was added with stirring and stirring was continued until complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). Suspended silica was vigorously stirred at -78°C (per 1 mmol substrate 15 g)), the resulting heterogeneous mixture was then transferred to a suspension in ethyl acetate, and the suspension was allowed to warm to room temperature. The resulting suspension was then filtered and extracted with dichloromethane (3×15 mL) for 3 times, washed 3 times with brine (3×40mL).Finally, the combined organic layer was washed with Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =10:3), the colorless oily product 2a was obtained with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com