High activity pet hydrolase mutant and its application
A hydrolytic enzyme and activity technology, applied in the field of genetic engineering, can solve the problem of low enzyme activity, achieve the effect of improving activity, good industrial application prospects, and improving degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
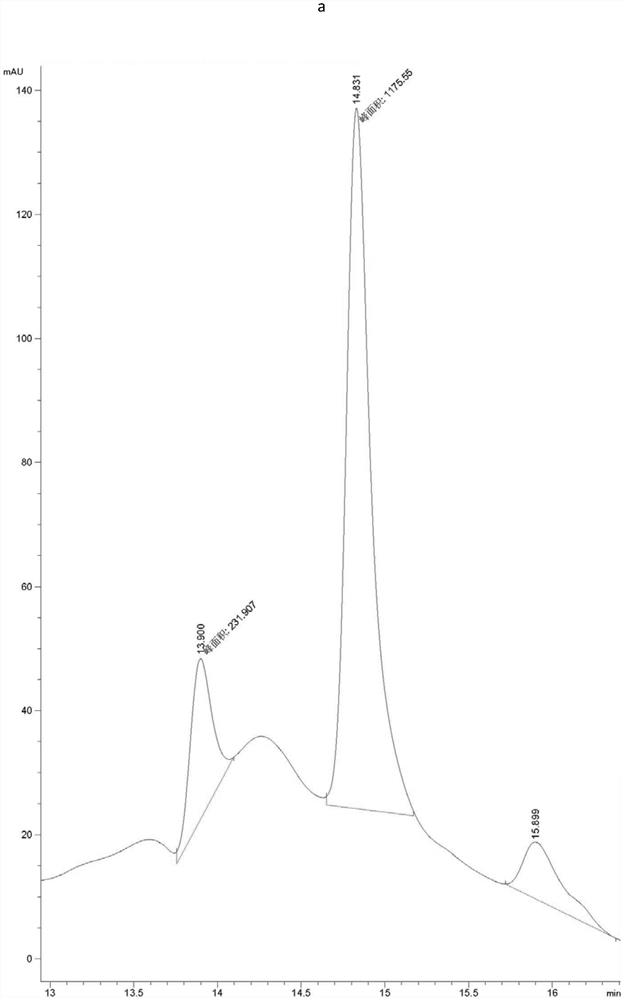
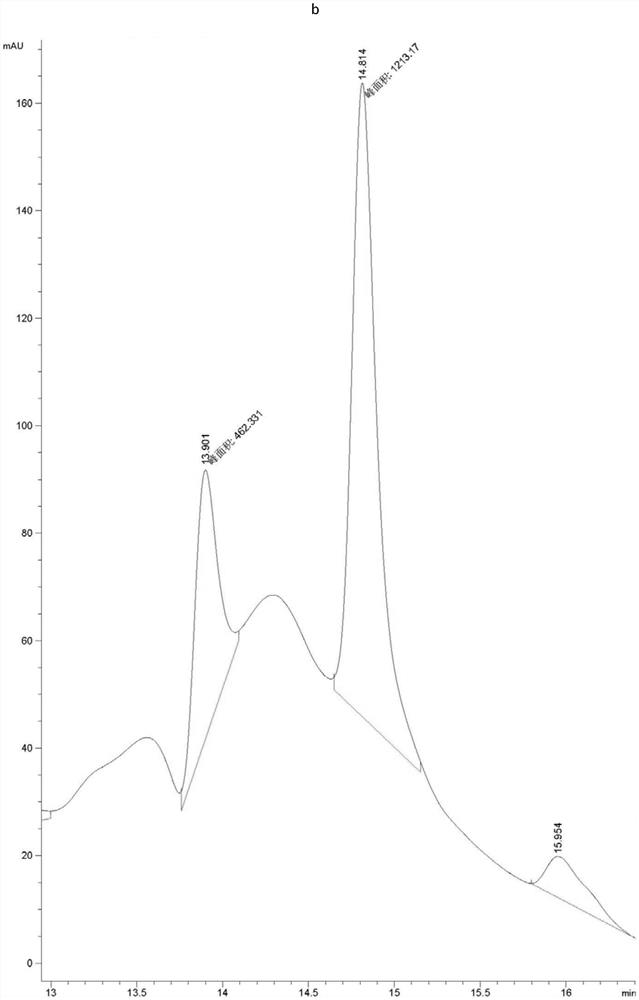
[0063] Example 1, Preparation, expression purification and activity detection of PET hydrolase mutants
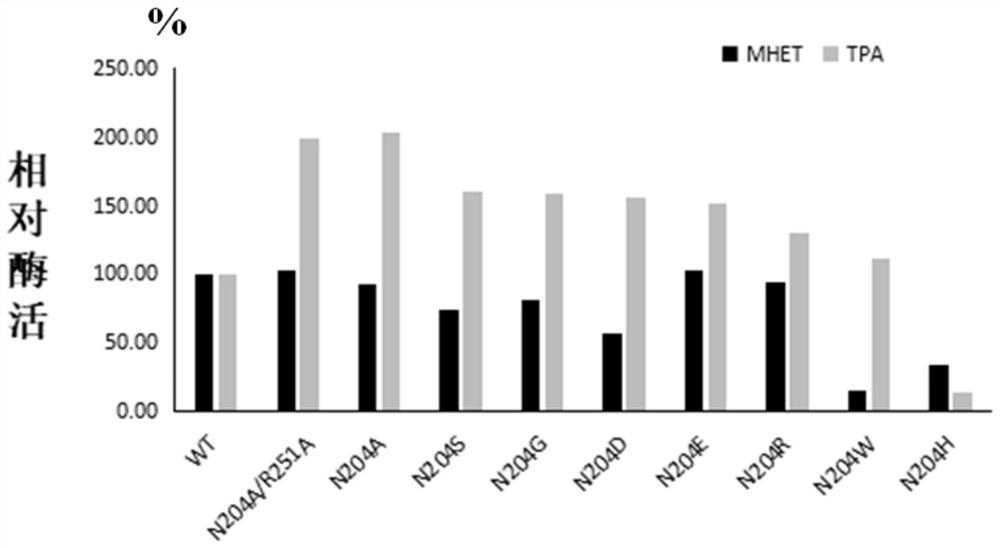
[0064] In order to increase the industrial application value of PET hydrolase, the present invention synthesized the gene of PET hydrolase derived from Ideonella sakaiensis, and expressed and purified it. After studying the structure of the PET hydrolase, its active region participated in the substrate The interacting amino acids were mutated to increase the activity of the enzyme towards the substrate PET.
[0065] 1. Construction of wild-type PET hydrolase recombinant plasmid and its mutant recombinant plasmid
[0066] 1. Construction of wild-type PET hydrolase recombinant plasmid
[0067] The wild-type PET hydrolase is PETase derived from Ideonella sakaiensis. The nucleotide sequence of the wild-type PET hydrolase is sequence 1 in the sequence listing, and the encoded amino acid sequence is sequence 2 in the sequence listing.
[0068] Insert the nucleotides of the wild...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com