Anti-SpA5 protein monoclonal antibody, application thereof, and kit containing monoclonal antibody
A technology of monoclonal antibody and kit, which is applied in the direction of anti-bacterial immunoglobulin, immunoglobulin, biological testing, etc. It can solve the problem that there is no specific anti-SpA5 antibody, and achieve the effect of high sensitivity and wide detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of mouse monoclonal antibody that specifically recognizes SpA5 protein
[0053] 1. Preparation and screening of monoclonal antibodies
[0054]SpA5 recombinant protein (1.4mg / ml) was mixed with adjuvant CFA and AD11.15 to prepare the immunogen, that is, the recombinant protein was first mixed with CFA at a volume ratio of 5:6 as immunogen A, and then the recombinant protein was mixed with AD11. 15 was mixed at a volume ratio of 1:1 as immunogen B. Immunogen A is the primary immunization and immunogen B is the booster immunization. Three mice were immunized intramuscularly, and the tail blood of the mice was drawn on the 14th day after immunization, and the antibody titer of the tail blood was evaluated by indirect ELISA method.
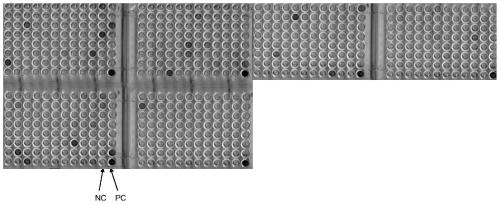
[0055] Coat the ELISA plate with SPA5 recombinant protein (1 μg / mL), add 100 μL to each well, react overnight at 4°C; wash the plate 3 times with PBS solution, block with 5% milk-PBS at room temperature for 1 hr; then wa...
Embodiment 2
[0078] Example 2: Preparation and use of double antibody sandwich ELISA detection kit for SpA5 protein
[0079] The preparation method of the rabbit polyclonal antibody against SpA5 protein: ① adjust the recombinant protein SpA5 solution to a concentration of 1mg / mL with diluent (10mMHis, 0.9%NaCl, pH=6.0), and mix with Freund’s Adjuvant (complete Freund's adjuvant for the first immunization, incomplete Freund's adjuvant for booster immunization) emulsified. ②The emulsified antigen was injected subcutaneously near the lymph nodes in the limbs of rabbits, 200 μL for each point, 0.4 mg in total. The immunization program was 0, 14, 21 days, a total of 3 times of immunization. ③ On the 28th day after the first immunization, 100 μL of blood was collected from the immunized rabbits through the ear vein, the serum was separated, and the antibody titer corresponding to the recombinant protein SpA5 was detected by indirect ELISA. ④ After the antibody titer corresponding to the recomb...
Embodiment 3
[0115] Embodiment 3: sample detection
[0116] Use the kit prepared in Example 2 to detect the protein content of SpA5 antigen in the 20180903 batches of finished products of Staphylococcus aureus vaccine. The detection steps are as follows:
[0117] 1. Sample pretreatment: Take 20 vaccine finished products, mix each tube, draw 600μL into a 1.5mL EP tube, centrifuge at 5000rpm for 10min, absorb 300μL of the supernatant, add 2mol / L Na 2 CO 3 300 μL of the solution and mix well, after the solution is clear, centrifuge to take an appropriate amount of supernatant for later use;
[0118] 2. according to the operation step of embodiment 2, sample is detected;
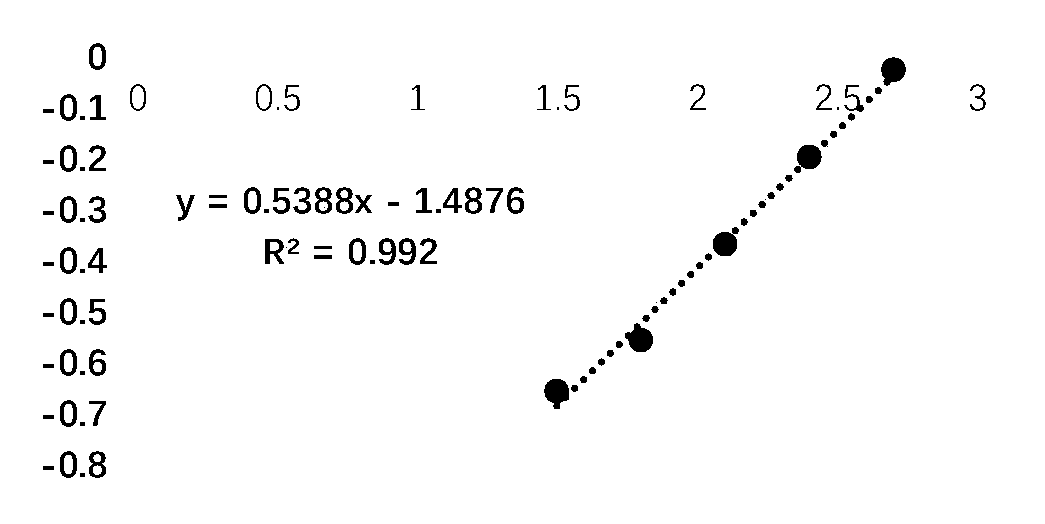
[0119] 3. The results of the standard were linearly fitted using the logX-LogY fitting method to obtain a standard curve, and the absorbance value (OD value) of the test sample was substituted into the standard curve to calculate the sample concentration. The test results are as follows:
[0120] Table 6: The results of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com