Application of Amurensin H derivative EAPP in treatment and prevention of aplastic anemia
An aplastic, derivative technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
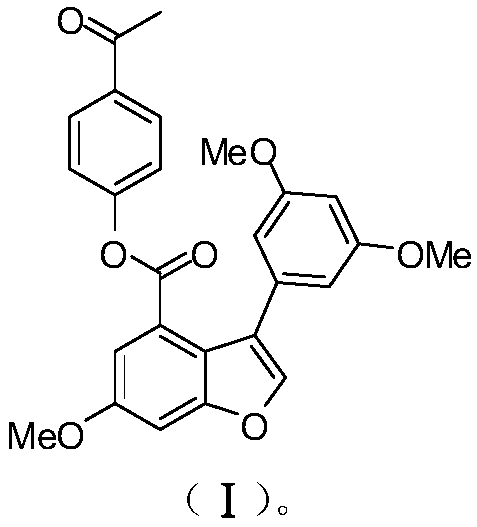
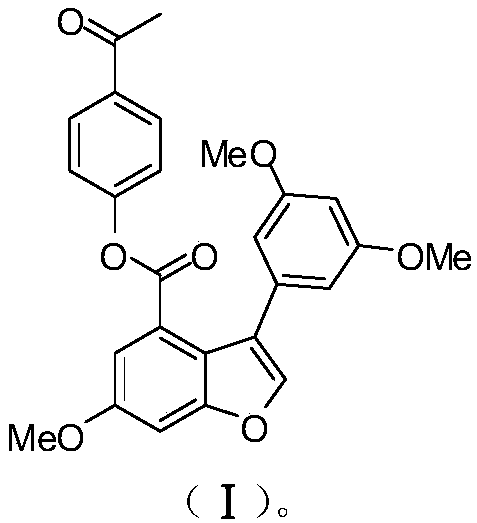
[0050] Example 1: Preparation of compound (I) [3-(3,5-dimethoxyphenyl)-6-methoxy-4-benzofurancarboxylic acid-4-acetylphenyl ester].
[0051] The synthetic steps of compound EAPP (I) are as follows:
[0052] Step 1: Synthesis of α-phenoxyketocarboxylic acid through etherification reaction between methyl 3-methoxy-5-hydroxybenzoate and 1-(3,5-dimethoxyphenyl)-2-bromoacetophenone methyl ester.
[0053] Methyl 3-methoxy-5-hydroxybenzoate (4.5g, 26.8mmol) was dissolved in 1-(3,5-dimethoxyphenyl)-2-bromoacetophenone (6.94g, 26.8mmol) In 150ml of dry acetone, slowly add K under vigorous stirring 2 CO 3 The solid was 7.39g (53.5mmol), and the reaction solution was stirred at room temperature for 3h, and then heated to reflux for 3h. TLC monitoring showed that the reaction of raw materials was complete. The reaction solution was cooled to room temperature, filtered with diatomaceous earth, washed with acetone, and the organic phase was concentrated under reduced pressure. The resu...
experiment example 1
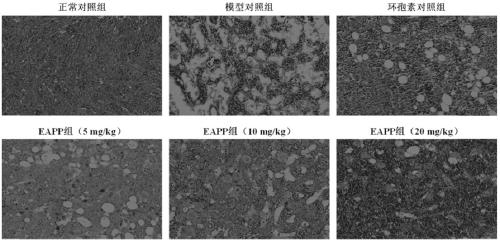
[0065] Experimental example 1: The effect of EAPP on aplastic anemia mouse model
[0066] experimental method:
[0067] Get male BALB / c mouse of 18-20g, divide into normal control group, model control group, positive control group (cyclosporine 10mg / kg, intragastric administration) at random, EAPP group (5mg / kg, 10mg / kg, 20mg / kg, intragastric administration). In addition to the blank control group, the animals in each group were inoculated intravenously with cobalt-60γ rays (5.80Gy cobalt-60γ rays for one whole body irradiation) + DBA / 2 mouse thymus:spleen immune cells (2:1) to prepare aplastic anemia models ; From the day of modeling, intragastric administration, once a day, for 14 consecutive days. One hour after the last administration, blood was collected from the orbital vein of the mice, and EDTA-K 2 Anticoagulation, five-differential blood cell analyzer to detect blood routine and flow cytometry to detect reticulocyte count; remove the thymus and spleen of the mouse,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com