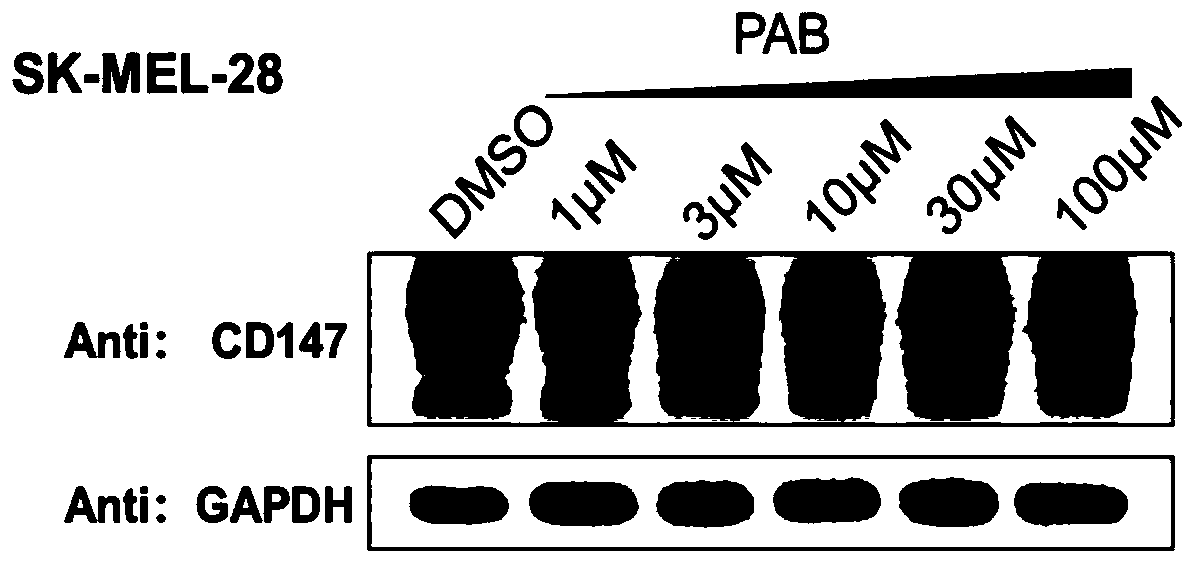
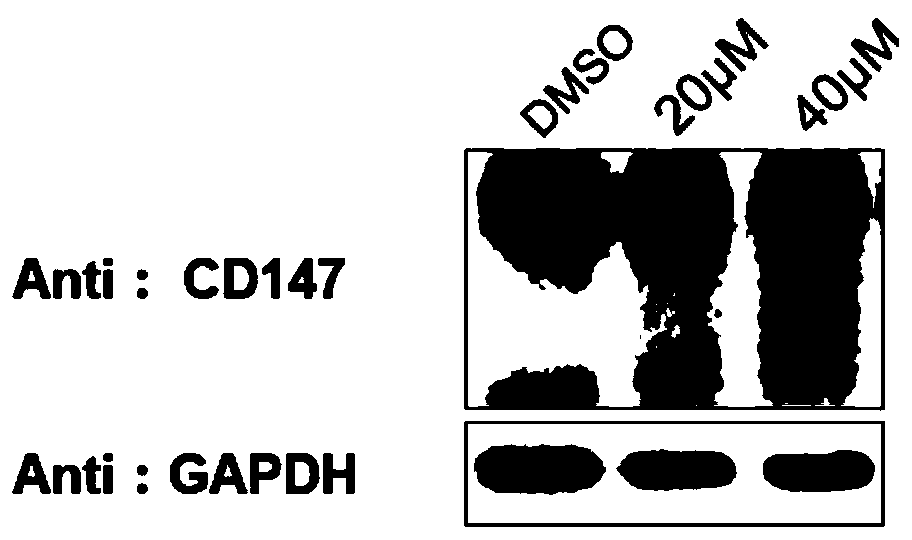
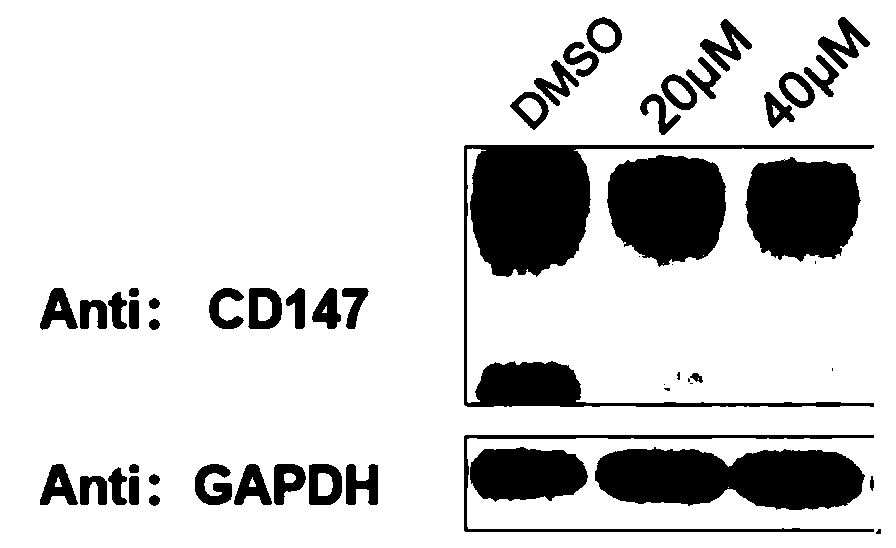
Bifunctional organic compound and preparation method and application thereof
An organic compound, dual-functional technology, applied in the direction of organic chemistry, drug combination, antineoplastic drugs, etc., to achieve the effect of inhibiting the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Further, one embodiment of the present invention provides a preparation method of the above-mentioned bifunctional organic compound, the preparation method comprising the following steps: dehydrating the compound (I-a) with the carboxyl group and the primary amino group, the compound (I-b) and the vitex acetic acid respectively Condensation reaction, to obtain bifunctional organic compounds.
[0055] Among them, the structures of compound (I-a) and compound (I-b) are as follows, wherein R is at least one selected from alkenyl, alkynyl, substituted or unsubstituted alkyl, substituted or unsubstituted alkoxy;
[0056] HOOC-R-NH 2 (I-a);
[0057]
[0058] Compound (I-b) (Protein degrader 1 hydrochloride, protein degrader 1 hydrochloride) is a small molecule ligand of Von-Hippel-Lindau (VHL) protein in the E3 ubiquitin ligase complex, which can bind to E3 ubiquitin Ligase specific binding.
[0059] In one of the embodiments, the structure of the above compound (I-a) is...
Embodiment 1
[0090] The structural formula of the bifunctional organic compound 1 is shown below:
[0091]
[0092] The synthesis steps are as follows:
[0093] 1) Dissolve Boc-glycine (21 mg, 0.12 mmol) in 3 mL of dichloromethane, and add 2-(7-benzotriazole oxide)-N, N, N', N'-tetramethylurea hexa Fluorophosphate (HATU, 64 mg, 0.17 mmol), N, N-diisopropylethylamine (59 μL, 0.36 mmol), compound (I-b) (Protein degrader 1 hydrochloride, 56 mg, 0.12 mmol), reacted for 12 hours, filtered Afterwards, the filtrate was spin-dried, and purified by column chromatography (DCM / MeOH volume ratio=20:1) to obtain 37 mg of intermediate 1b. The structure of intermediate 1b is shown below.
[0094]
[0095] The characterization results of intermediate 1b are as follows:
[0096] 1 HNMR (500MHz, CDCl 3 )δ8.68 (s, 1H), 7.37-7.32 (m, 4H), 4.55-4.45 (m, 3H), 4.41-4.36 (m, 1H), 4.02 (d, J=11.1Hz, 1H), 3.83 -3.63(m, 3H), 2.51(s, 3H), 2.35-2.15(m, 2H), 1.49-1.36(m, 13H), 0.97(s, 9H).
[0097] HRMS(ESI...
Embodiment 2
[0104] The structural formula of the bifunctional organic compound 2 is shown below:
[0105]
[0106] The synthesis steps are the same as in Example 1, except that the Boc-glycine in step 1) of Example 1 is converted into compound 2a to obtain intermediate 2b. The structures of compound 2a and intermediate 2b are shown below.
[0107]
[0108] The characterization results of intermediate 2b are as follows.
[0109] 1 H NMR (500MHz, CDCl 3 )δ: 8.69 (s, 1H), 7.43-7.32 (m, 5H), 6.38-6.28 (m, 1H), 4.78-4.67 (m, 2H), 4.62-4.49 (m, 3H), 4.33 (dd, J=15.0, 5.2Hz, 1H), 4.11(d, J=11.2Hz, 1H), 3.60(d, J=10.1Hz, 1H), 3.12-3.01(m, 2H), 2.51(s, 3H), 2.29-2.12(m, 3H), 1.68-1.56(m, 2H), 1.53-1.33(m, 12H), 0.93(s, 9H).
[0110] HRMS (ESI) m / z: calcd for C 32 h 48 N 5 o 6 8(M+H) + :630.3320, found: 630.3326.
[0111] The characterization results of bifunctional organic compound 2 are as follows:
[0112] 1 HNMR (500MHz, CDCl 3 )δ: 8.70 (s, 1H), 7.43-7.33 (m, 5H), 7.23-7.18 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com