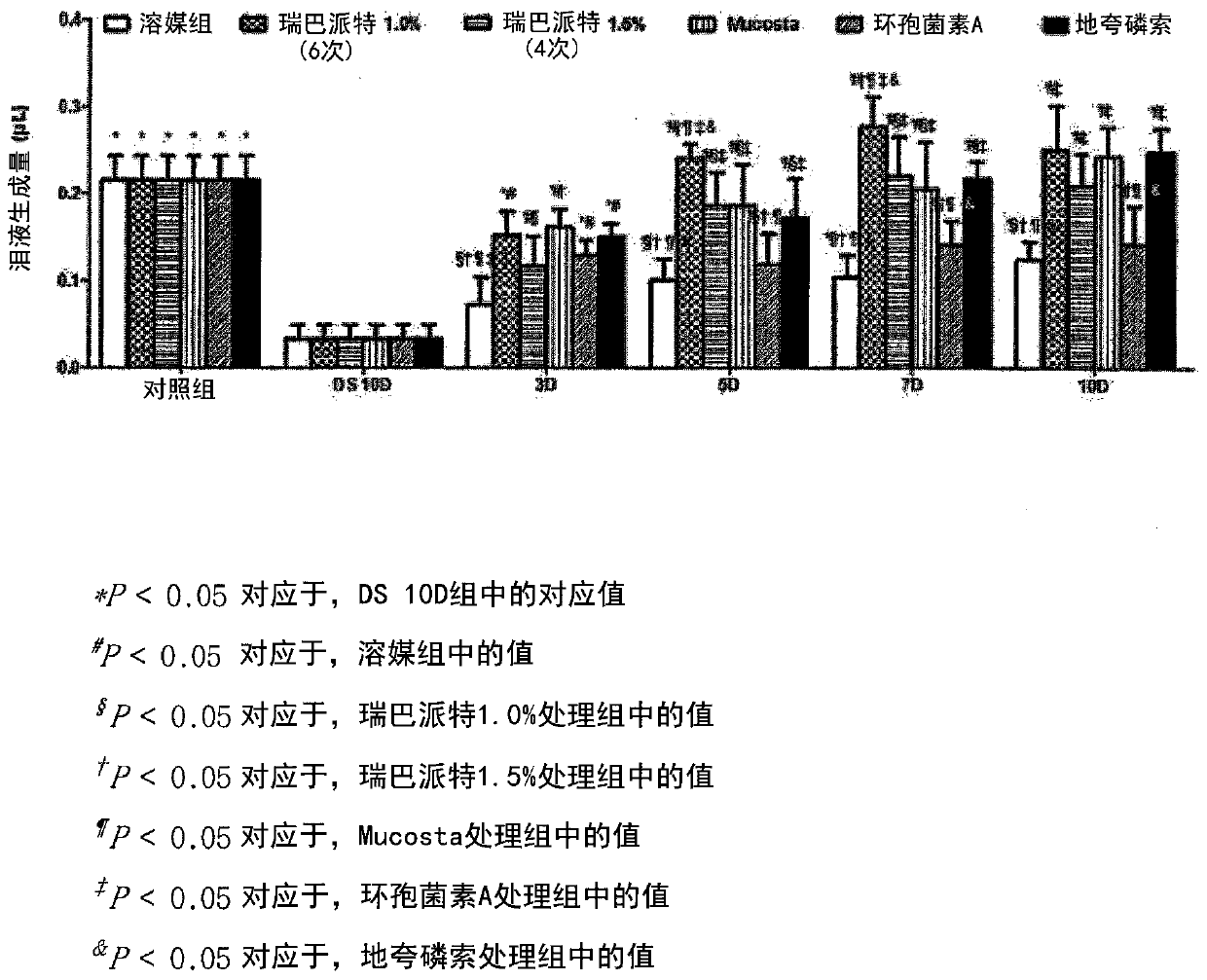
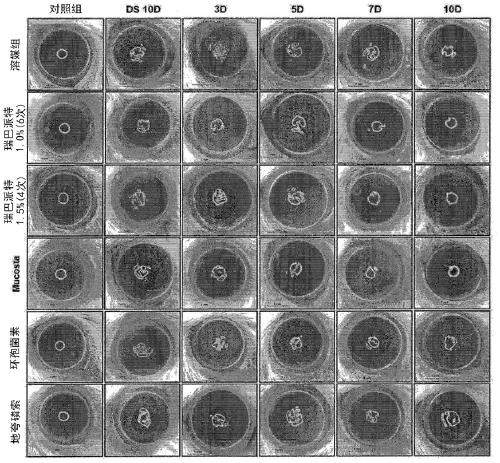
Novel eye drop composition for treating dry eye syndrome containing rebamipide and method for solubilizing and stabilizing same
The technology of a special eye drop, rebamipide, is applied in the field of preparation of eye drop aqueous solution and its composition, which can solve problems such as biocompatibility and achieve minimal side effects, convenient use, and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9 to Embodiment 18
[0126] [Example 9 to Example 18] Preparation of 1.5% eye drops by solubilizing rebamipide
[0127] [table 5]
[0128]
[0129]
[0130] 1) Add the stabilizer corresponding to each example in 85ml of distilled water and stir to dissolve, then add the main component rebamipide and stir to suspend it.
[0131] 2) Add 1N NaOH solution as a solubilizer dropwise to the solution of 1) under stirring, adjust the pH to 10-11, and dissolve all the main components.
[0132] 3) Add the buffer corresponding to each example to the solution of 2) and stir to dissolve them all.
[0133] 4) Add the osmotic pressure regulator corresponding to each example to the solution of 3) and stir to adjust to a hypotonic range of 220 to 240 mOsmol / kg that can be used as eye drops.
[0134] 5) Add dropwise the 1N HCl solution corresponding to each embodiment as a pH regulator to the transparent solution obtained in the above 4), and adjust the pH to 7-8.
[0135] 6) Add distilled water to the tran...
Embodiment 19 to Embodiment 22
[0147] [Example 19 to Example 22] Preparation of 1.5% eye drops by solubilizing rebamipide
[0148] [Table 7]
[0149]
[0150]
[0151] 1) Add a stabilizer to 85ml of distilled water and stir to dissolve, then add the main component rebamipide and stir to suspend it.
[0152] 2) Add 1N NaOH solution as a solubilizer dropwise to the solution of 1) under stirring, adjust the pH to 10-11, and dissolve all the main components.
[0153] 3) Add the buffer corresponding to each example to the solution of 2) and stir to dissolve them all.
[0154] 4) Add the osmotic pressure regulator corresponding to each embodiment to the solution of 3) and stir to adjust to a hypotonic 170-190 mOsmol / kg.
[0155] 5) Add dropwise the 1N HCl solution corresponding to each embodiment as a pH regulator to the transparent solution obtained in the above 4), and adjust the pH to 7-8.
[0156] 6) Add distilled water to the transparent solution obtained in 5) to make the total volume 100 ml to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com