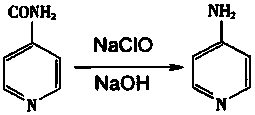
Preparation method and post-treatment process of 4-aminopyridine
An aminopyridine and process technology, which is applied in the field of chemical raw material synthesis, can solve the problems of high requirements on reaction conditions, high catalyst costs, and low yields, and achieve the effects of mild reaction conditions, easy control, simple post-treatment methods, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method and post-treatment process of 4-aminopyridine, the steps of which are:
[0032] S1: Using isonicotine as a raw material, carry out Hoffman degradation reaction in sodium hypochlorite solution and 10% sodium hydroxide solution; first pour 195g of sodium hypochlorite solution into a four-necked flask (wherein the sodium hypochlorite solution has an available chlorine content of 6 %), the four-neck flask was placed in a 0°C cold trap to cool down while stirring was started. When the temperature of the sodium hypochlorite solution in the four-necked flask drops below 10°C, 20 g of solid isonicotine is added at this time, and after stirring until clarification, the stirring reaction is continued. Test the pH value and use HPLC to detect whether the isonicotine is completely converted into an intermediate, then add 10g of 10% sodium hydroxide solution to adjust the feed liquid to strong alkalinity, then transfer it to a water bath to slowly heat up, keep ...
Embodiment 6
[0039] A preparation method and post-treatment process of 4-aminopyridine, the steps of which are:
[0040] S1: Using isonicotine as raw material, carry out Hoffmann degradation reaction in sodium hypochlorite solution and 10% sodium hydroxide solution; first, pour 580g of sodium hypochlorite solution into a four-necked flask (among them, the sodium hypochlorite solution has an available chlorine content of 7.5 %), the four-neck flask was placed in a 0°C cold trap to cool down while stirring was started. After the temperature of the sodium hypochlorite solution in the four-neck flask dropped below 10°C, 75 g of solid isonicotine was added at this time, and after stirring until clarified, the stirring reaction was continued. Test the pH value and use HPLC to detect whether the isonicotine is completely converted into an intermediate, then add 30g of 10% sodium hydroxide solution to adjust the feed liquid to strong alkalinity, then transfer it to a water bath to slowly heat up, ...
Embodiment 8
[0047]S1: Using isonicotine as a raw material, carry out Hoffmann degradation reaction in sodium hypochlorite solution and 10% sodium hydroxide solution; first pour 365g of sodium hypochlorite solution into a four-necked flask (wherein the sodium hypochlorite solution has an available chlorine content of 12 %), the four-neck flask was placed in a 0°C cold trap to cool down while stirring was started. After the temperature of the sodium hypochlorite solution in the four-neck flask dropped below 10°C, 75 g of solid isonicotine was added at this time, and after stirring until clarified, the stirring reaction was continued. Test the pH value and use HPLC to detect whether isonicotine is completely converted into an intermediate, then add 19g of 10% sodium hydroxide solution to adjust the feed liquid to strong alkalinity, then transfer it to a water bath to slowly heat up, keep warm for a certain period of time, and set a certain time during the reaction. Samples were taken for HPL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com