COMPOSITIONS AND METHODS RELATED TO xCT ANTIBODIES
A technology of antibodies and antibody fragments, applied in the fields of YFTTI, QTQNFKDAFSGRDSSITRLP, can solve the problems of SASP instability, off-target effects, low bioavailability, and insolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0071] In one embodiment, according to the present invention "K D " or " K D "values" are measured by radiolabeled antigen binding assays (RIA) performed with antibodies and Fab forms of antigen molecules, as described in the following assay, by titrating a series of unlabeled antigens with minimal concentrations of ( 125 1) Labeled antigen equilibrates Fab, then captures bound antigen with anti-Fab antibody-coated plates to measure solution binding affinity of Fab to antibody (Chen et al., (1999) J. Mol. Biol 293:865-881). To establish assay conditions, microtiter plates (Dynex) were coated overnight with 5 μg / ml capture anti-Fab antibody (Cappel Labs) in 50 mM sodium carbonate (pH 9.6) and then washed with 2 wt. BSA solution in PBS / vol% for two to five hours of blocking. In non-binding plates (Nunc#269620), 100pM or 26pM [ 125 I] Antigen is mixed with serial dilutions of Fab of interest. The Fab of interest is then incubated overnight. However, the incubation may be con...
Embodiment 1
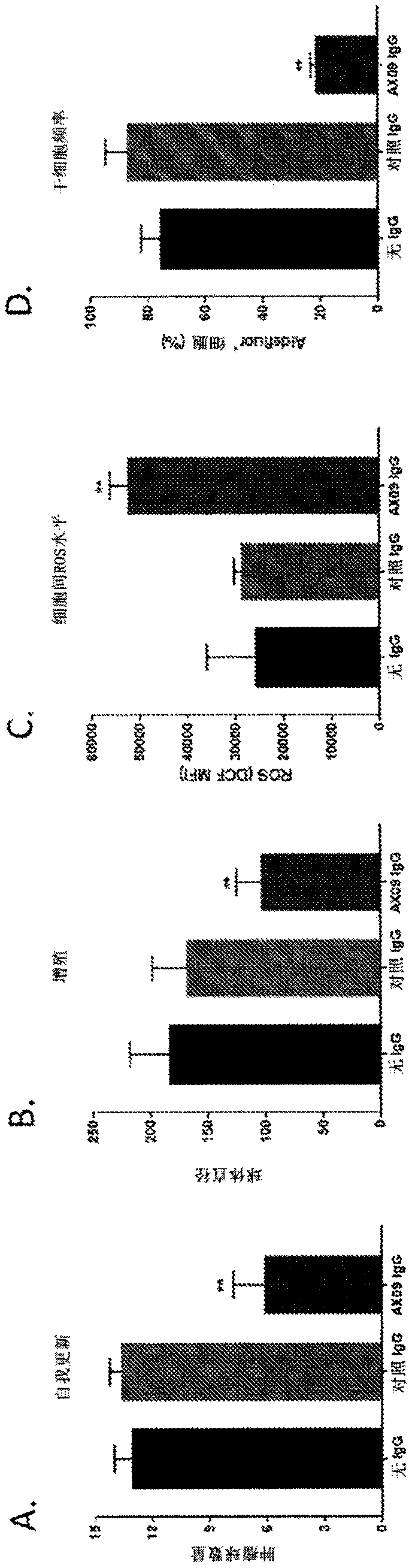
[0146]To investigate whether antibodies induced by xCT VLPs affect xCT function and BCSC biology, IgG was purified from serum of control VLP or xCTVLP-treated mice. BCSC-enriched cells from the indicated sTNBC cell lines were incubated with purified antibodies, and 3D cultures were used to analyze BCSC self-renewal and proliferation, while the concentration of aldefluor-positive cells (a marker of BCSC phenotype) in spheroids was measured by FACS. Frequency and intercellular ROS levels (indicators of xCT function). As observed with other methods of xCT inactivation (siRNA, SASP treatment and DNA vaccination), xCT VLP-induced IgG antibodies reduced BCSC self-renewal, proliferation and increased ROS levels ( figure 1 ). The number of BCSCs was also significantly reduced in tumorspheres formed in cultures treated with xCT VLP-induced antibodies.
[0147] figure 1 showed that AX09 (an xCT epitope displayed on RNA phage VLP)-induced antibodies affects BCSC biology and inhibits...
Embodiment 2
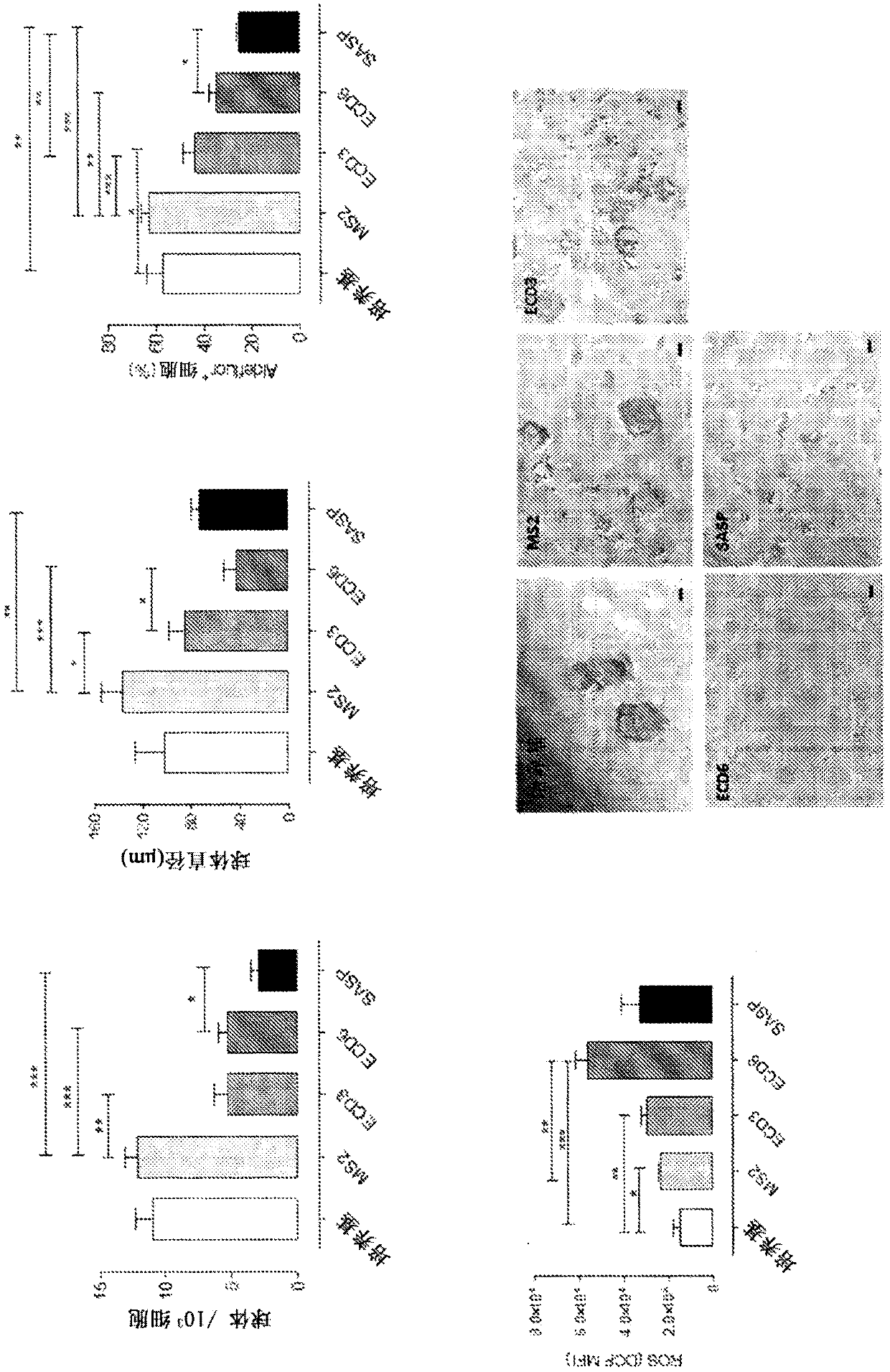
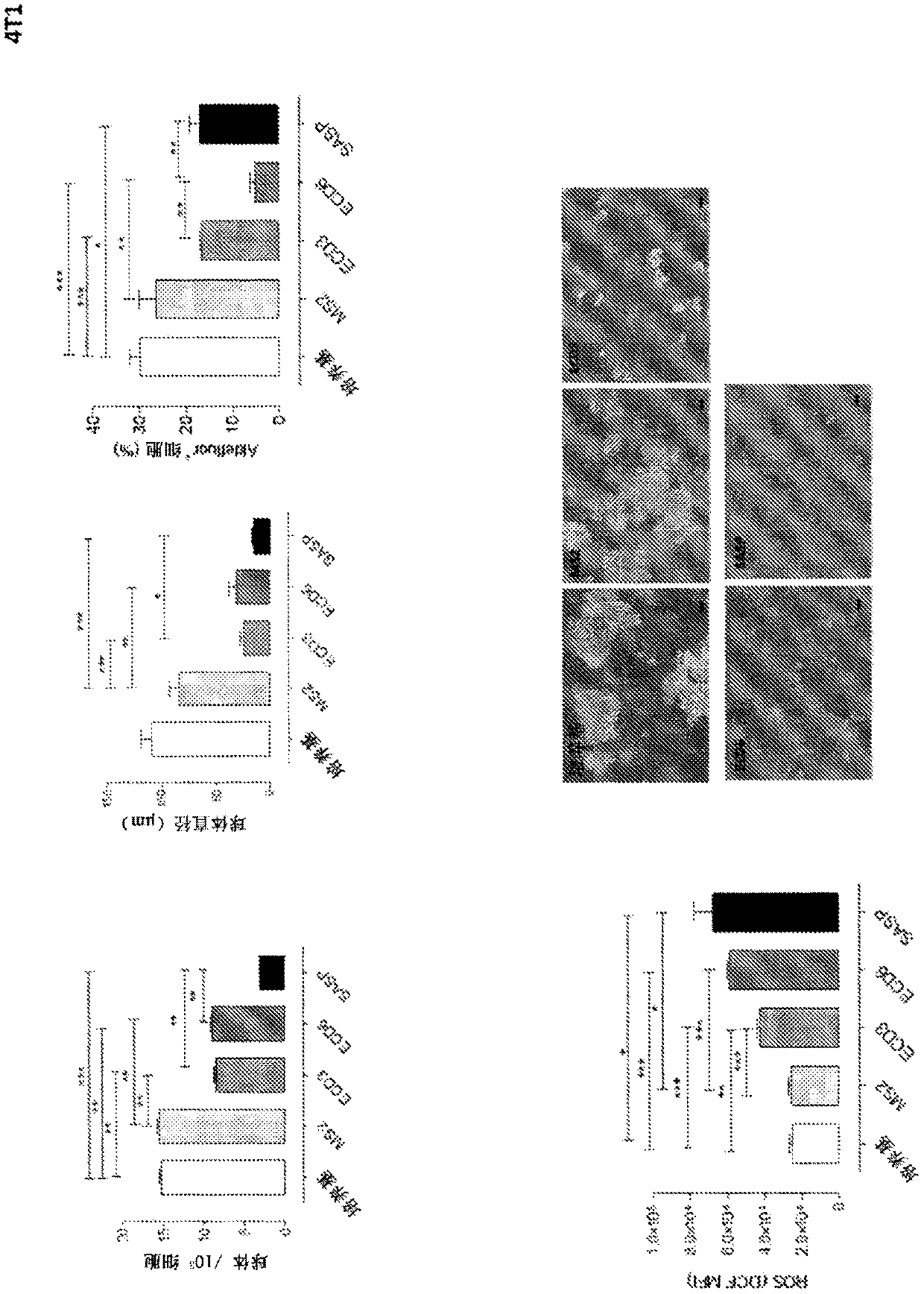
[0207] Generation of monoclonal antibodies against xCT. Female BALB / c mice (Charles River Laboratory) were kept at the Center for Molecular Biotechnology, University of Turin, and were handled in accordance with the University Ethics Committee and European Guidelines 2010 / 63. Vaccination involved an intramuscular electroporation of the pVAX1-xCT DNA plasmid (encoding full-length mouse xCT protein), as previously described (Lanzardo et al., 2016). Starting on day 10 after DNA vaccination, mice were boosted 6 times per month with AX09-0M3 VLP (anti-third extracellular domain of human xCT protein; ECD3). Mice were bled before the first VLP vaccination and then two weeks after each vaccination, and serum was collected and stored at −20°C for subsequent analysis.
[0208] To test the functional effect of vaccination-induced antibodies, 4T1-derived tumorspheres enriched in xCT+ cancer stem cells (CSCs) were treated with medium alone, the xCT pharmacological inhibitor sulfasalazine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com