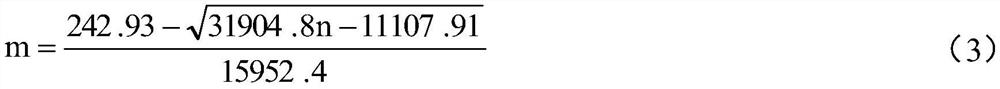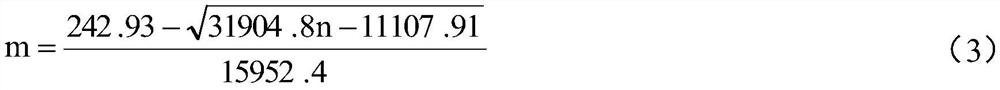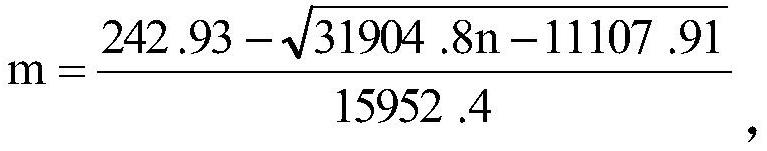A luminescent bacteria freeze-dried powder kit and preparation method thereof
A technology of luminescent bacteria and lyophilized powder, applied in biochemical equipment and methods, bacteria, microorganism-based methods, etc., can solve the problems of poor reproducibility of the kit and the regulation of important parameters of the kit without mentioning it, and achieve consistent performance. The effect of stability, CF value and negative control value, and positive control value stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This kit ensures the stability of the initial luminescence amount through the luminescence amount adjustment method, ensures the stability of the positive control value through the positive control value adjustment method, and ensures the CF value and negative control value of the lyophilized powder kit by stabilizing the entire production process. value stability.
[0061] The production process of the kit includes three steps: the preparation of the freeze-dried powder of the strain, the preparation of the freeze-dried powder of the kit, and the preparation of the supporting liquid reagent. To ensure the stability of the bacteria during re-inoculation in the subsequent production process. In the preparation of lyophilized powder of the kit, the adjustment of the amount of the prepared bacterial liquid and the composition of the protective agent effectively ensures the stability of the initial luminescence amount and positive control value of the production kit. At th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com