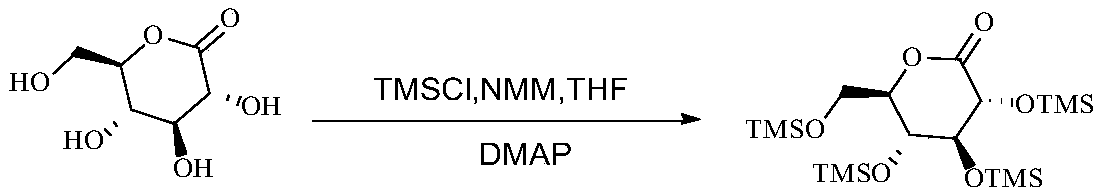
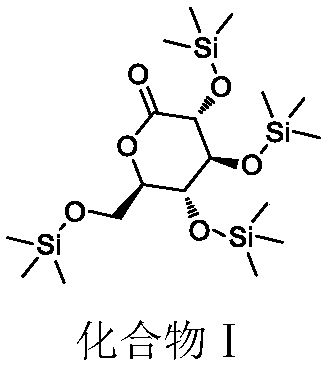
Preparation method of 2, 3, 4, 6-tetra-O-trimethylsilyl-D-glucolactone
A technology of gluconolactone and trimethylsilyl, applied in the field of preparation of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone, which can solve the problem of cooling production equipment Strict efficiency requirements, unstable product quality and production, etc., to achieve the effect of lower cooling efficiency requirements, stable reproducibility, and easy operation of the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 400L tetrahydrofuran and 290kg (2.86kmol, 6.4eq) N-methylmorpholine to the reaction flask, cool down to -5~15°C, slowly add 250kg (2.3mol, 5.1eq) trimethylchlorosilane dropwise, control system -5~15°C, after dropping, add 80kg (449.4mol, 1.0eq) gluconolactone in batches, and control the temperature at -5~20°C; add 1.4kg (23mol, 0.05eq) DMAP after feeding, and then Raise the temperature to 30-35°C and react for 3-5 hours; add 400kg of toluene, cool down to 0-10°C, add dropwise 100L of aqueous solution to quench the temperature at 0-10°C, add 250L of water to dissolve the system, and remove the water phase. The aqueous phase was extracted with 100L of toluene and the organic phase was combined. The organic phase was washed with 540 kg of 7% sodium dihydrogen phosphate aqueous solution and 520 kg of saturated brine. 207.6 kg of yellow oil, yield 99.1%, GC purity 98.7%, impurity compound IV purity 0.11%.
Embodiment 2
[0044] Add 200ml tetrahydrofuran and 79.7g (787.5mmol, 7.0eq) N-methylmorpholine to the reaction flask, cool down to 15-30°C, slowly add 73.3g (675mmol, 6.0eq) trimethylchlorosilane dropwise, control system 15-30°C, after dropping, add 20g (112.5mmol, 1.0eq) gluconolactone in batches, and control the temperature at 20-40°C; add 0.27g (2.25mmol, 0.02eq) DMAP after the addition, and then raise the temperature React at 30-35°C for 3-5 hours; add 100g of toluene, cool down to 5-15°C, add dropwise 25ml of aqueous solution to quench the temperature at 5-15°C, add 62.5ml of water to dissolve the system, and remove the water phase. The aqueous phase was extracted with 25ml of toluene and the organic phase was combined. The organic phase was washed with 135g of 7% sodium dihydrogen phosphate aqueous solution and 130g of saturated brine. The oil was 52.15g, the yield was 99.5%, the GC purity was 98.3%, and the impurity compound IV had a purity of 0.16%.
Embodiment 3
[0046] Add 200ml tetrahydrofuran and 145g (1.43mol, 6.4eq) N-methylmorpholine to the reaction flask, cool down to 20-30°C, slowly add 125g (1.15mol, 5.1eq) trimethylchlorosilane dropwise, control system 25 ~40°C, after dropping, add half of 20g (112.6mmol, 0.5eq) gluconolactone in batches, control the temperature at 25-40°C and stir overnight, then add the other half of 10g (112.6mmol, 0.5eq) gluconolactone Esters; add 1.4g (11.5mmol, 0.05eq) DMAP after feeding, then heat up to 30-35°C and react for 3-5h; add 200g toluene, cool down to 5-15°C, add dropwise 50ml of aqueous solution to quench control 5 ~15°C, add 125ml of water to dissolve the system, separate the water phase, extract the water phase with 50ml of toluene and combine the organic phase, wash the organic phase with 270g of 7% sodium dihydrogen phosphate aqueous solution, and wash with 260g of saturated saline , the organic phase was overbasic with alumina and then concentrated under reduced pressure to obtain 104.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com