A kind of preparation method of 16-alkene-17-ketoestrone derivative
A technology for ketoestrone and derivatives, which is applied in the field of preparation of 16-alkene-17-ketoestrone derivatives, and achieves the effect of easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
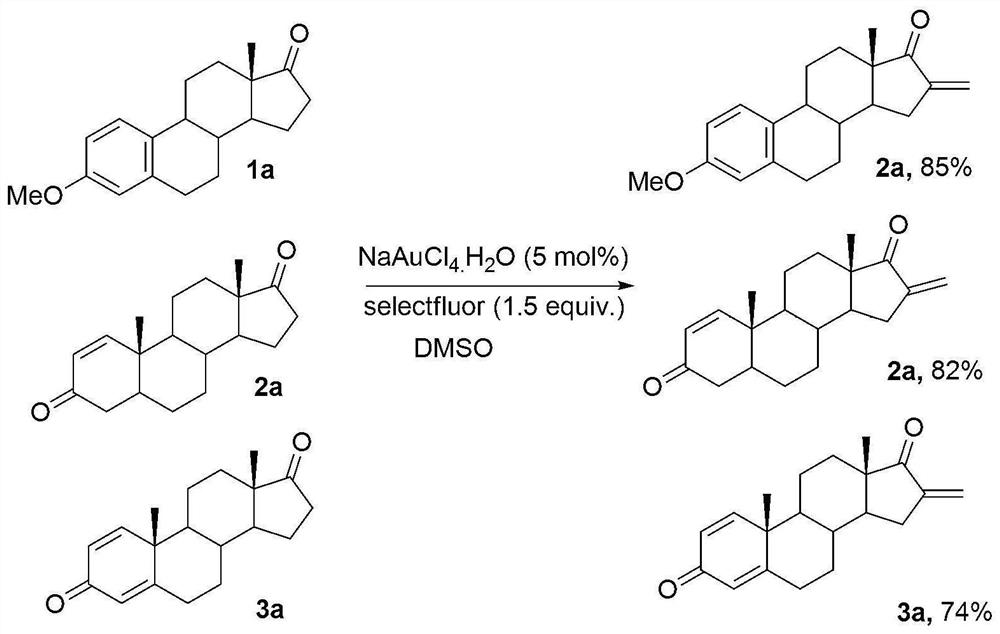
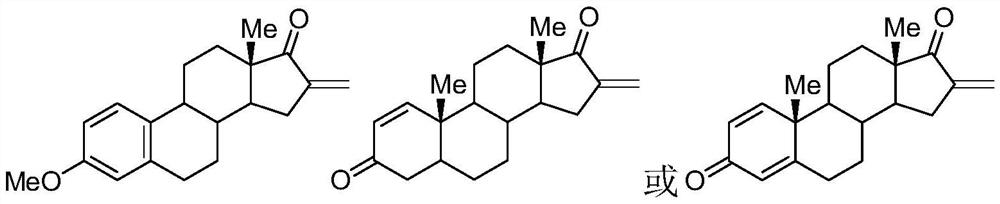
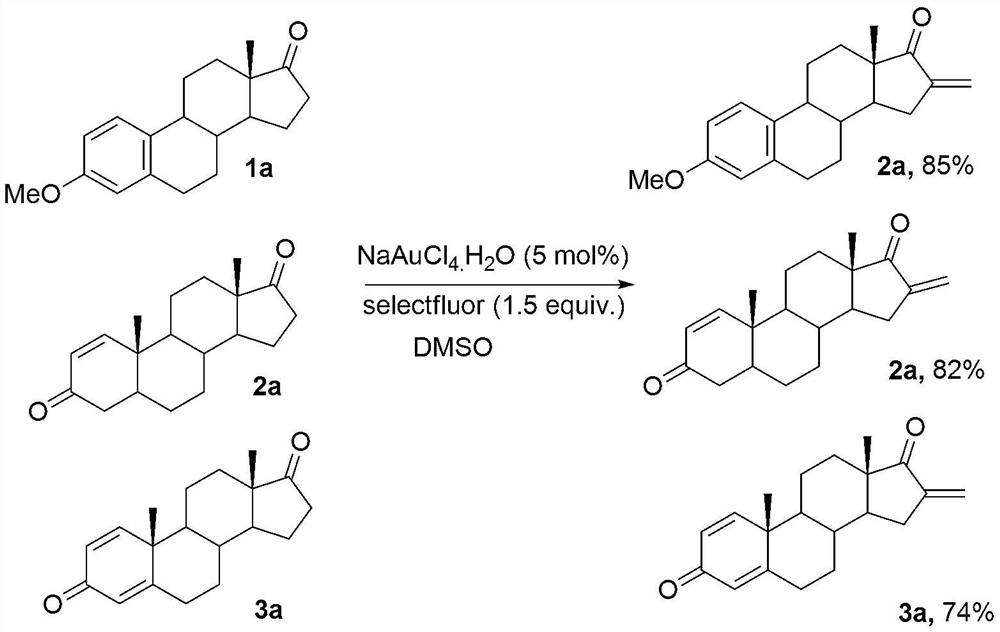
[0021] 3-Methylestrone (1mmol), fluorine reagent (2mmol), NaAuCl 4 (5mmol%) and 2mL DMSO were added to a 15mL test tube, heated to 100°C, reacted for 8 hours, and separated by silica gel column chromatography to obtain 3-methyl 16-alkene-17-ketoestrone 3a with a yield of 85%. M.p.121-123℃. 1 H NMR (400MHz, CDCl 3 )δ7.54(d,J=7.4Hz,2H),6.48-6.43(m,1H),6.26(s,1H),6.13(s,1H),3.82(s,3H),1.45–3.08(m ,13H),1.04(s,3H).
Embodiment 2
[0023] Androstenedione (1mmol), fluorine reagent (2mmol), NaAuCl 4. h 2 O (5mmol%) and 2mL DMSO were added to a 15mL test tube, heated to 100°C, reacted for 6 hours, and separated by silica gel column chromatography to obtain 16-alkene-17-ketoandrostenedione 3b with a yield of 82%. M.p.136-138℃. 1 H NMR (400MHz, CDCl 3 )δ7.86(d,J=7.4Hz,1H),6.26(s,1H),6.13(s,1H),6.04-5.98(m,1H),3.06-3.04(m,1H),2.85-2.83 (m,1H),2.19-1.48(m,14H),1.28(s,3H),1.15(s,3H).
Embodiment 3
[0025] 1,4-androstenedione (1mmol), fluorine reagent (2mmol), NaAuCl 4. h 2 O (5mmol%) and 2mL DMSO were added to a 15mL test tube, heated to 100°C, reacted for 8 hours, and separated by silica gel column chromatography to obtain the product of 16-alkene-17-keto-1,4-androstenedione 3c The rate is 74%. M.p.110-112℃. 1 H NMR (400MHz, CDCl 3 )δ6.98(d,J=8.2Hz,1H),6.42(d,J=7.2Hz,1H),6.28(s,1H),6.12(s,1H),2.10-1.48(m,6H), 1.39(s,3H),1.28(s,3H),1.32-1.18(m,8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com