Virus vector for expressing recombinant human beta-globin and application of virus vector
A technology of recombinant virus and globin, applied in the field of biomedicine, can solve the problem of cost-limiting the clinical application of gene therapy technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1, the determination of HBB expression module gene sequence
[0109] 1. Optimization of HBB expression cassette sequence
[0110] The mRNA sequence (accession number: NM_000518.5) and gene sequence (GeneID: 3043) of natural human HBB were obtained from the NCBI website database (https: / / www.ncbi.nlm.nih.gov / ). The natural HBB expression frame (exon) sequence was extracted from the NM_000518.5 sequence, and the sequence was codon-optimized on the website http: / / sg.idtdna.com / site to obtain the gene-optimized HBB exon sequence. The optimized HBB exon sequence of the gene was used to replace the natural human HBB exon sequence, and at the same time, the 374 bp sequence between the PstI sites in the natural human HBB intron II was deleted to obtain an optimized HBB expression cassette. The polynucleotide sequence of the optimized HBB expression cassette is as 2046-3277 of SEQ ID No.1, 2944-4175 of SEQ ID No.2, 1486-2717 of SEQ ID No.3 or SEQ ID The 308th-1539th...
Embodiment 2
[0129] Example 2, construction and virus packaging of lentivirus and adeno-associated virus vectors overexpressing HBB
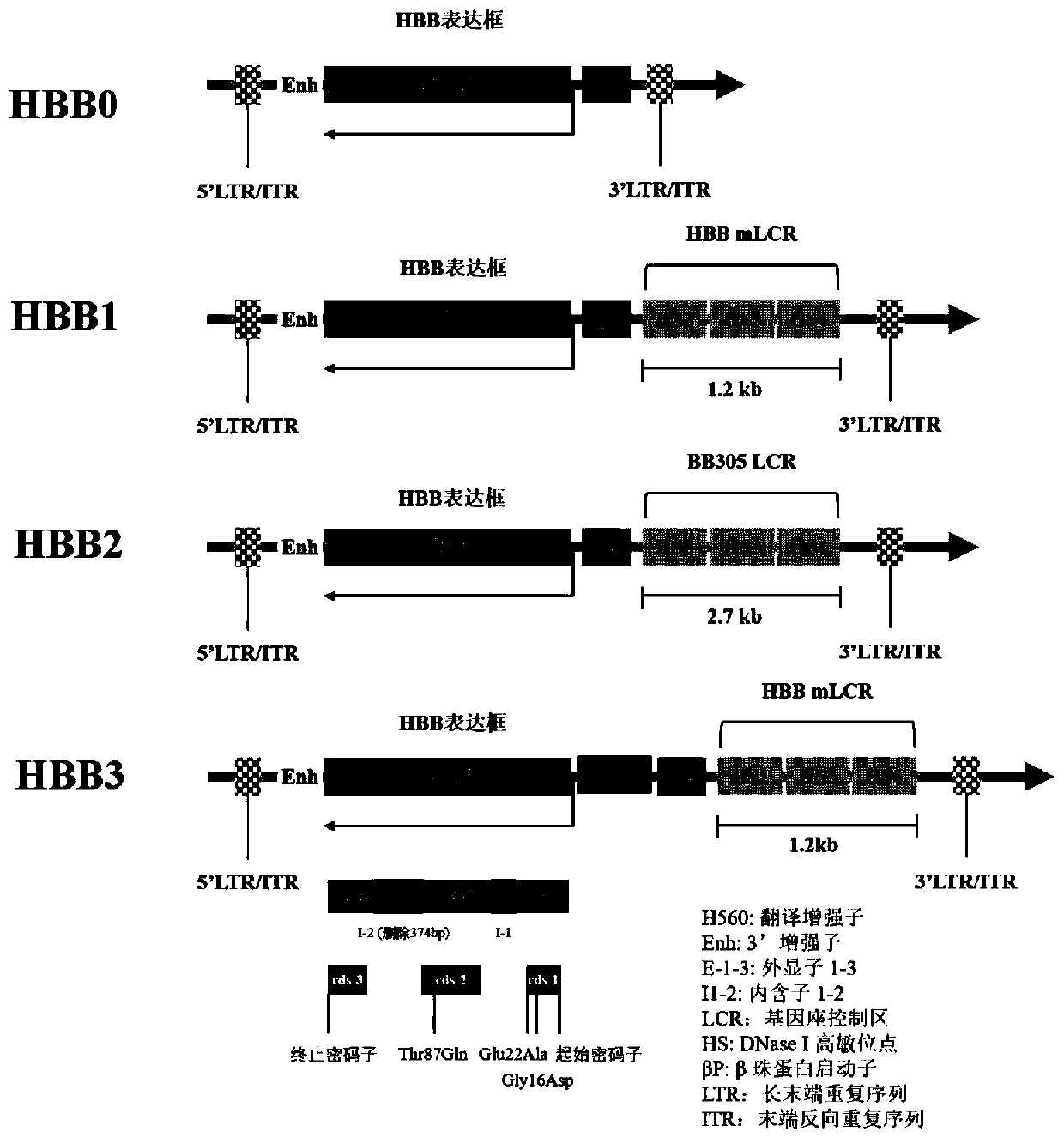
[0130] Add EcoRV and XbaI restriction sites at the ends of the above HBB0, HBB1, HBB2, and HBB3 sequences, respectively, and connect them to the lentiviral vector pLV-eGFP (Addgene) ( Figure 4 ) between the EcoRV and XbaI sites to obtain pLV-HBB0, pLV-HBB1, pLV-HBB2, and pLV-HBB3. In addition, a NotI restriction site was added at the end of the HBB0, HBB1 and HBB3 sequences, and connected to the adeno-associated virus vector pAAV-MCS ( Figure 5 ), pAAV-HBB0, pAAV-HBB1, and pAAV-HBB3 were obtained. The above ligation product was transformed into competent Escherichia coli (DH5α). Use Qiagen's endotoxin-free plasmid purification kit to extract and purify the plasmid, and the obtained plasmid can be used for lentivirus and adeno-associated virus packaging experiments after being verified by sequencing. In addition, pLV-eGFP and pAAV-GFP, which replaced the...
Embodiment 3
[0141] Embodiment 3, virus titer determination
[0142] For lentivirus, the virus titer was detected by flow cytometry, while for adeno-associated virus, the virus titer was detected by Q-PCR. The difference is that the viral titer of lentivirus is usually expressed by the number of active units per milliliter of virus fluid (TU / ml), while adeno-associated virus is usually expressed by the number of virus physical particles per milliliter of virus fluid (v.g / ml).
[0143] The specific experimental operation method of virus titer determination of lentivirus :
[0144] 1. HT1080 cells according to 1.5×10 5 Cells / 3ml / well were seeded into 6-well plates. Incubate at 37°C for 24h.
[0145] 2. Aspirate and discard the original medium, add 0.5ml DMEM complete medium (containing 8μg / ml polybrene) containing 5% FBS, and add concentrated pLV-eGFP virus solution dropwise according to the gradient of 0, 6.25, 12.5, 25, 50, and 100μl . Incubate at 37°C for 1 hour, add 2ml of culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com