Absolute quantitative PCR method for rapidly determining titer of ascoviruses
An absolute quantitative, virus titer technology, applied in biochemical equipment and methods, microbial determination/inspection, resistance to vector-borne diseases, etc. and other problems, to achieve the stability of experimental results, improve detection efficiency, and reduce time-consuming effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Primer Design
[0026] According to the publicly published vesicular virus HvAV-3h sequence, a single-copy region on the genome was selected to design specific primers, and the designed primers were screened to obtain a set of primer pairs with better specificity, stability and higher sensitivity. The nucleotide sequence is as follows:
[0027] AV-F: ATGAAAGCCGTGCTTAACGT (SEQ ID NO. 1),
[0028] AV-R: CTAGCCGGTGTGGTGGGTGT (SEQ ID NO. 2);
[0029] Using vesicle virus-infected cell genome and AV standard as template, the amplified sequence is:
[0030] ctagccggtgtggtgggtgtcgtatgtaatgcagttcgtcagaatgtctctaccgaagataatggtgtgtgtttcactattctcactctggaaaagcctccgcgtattagcgatcagaacgttaagcacggctttcat (SEQ ID NO. 3); the length of the amplified fragment is 138 bp.
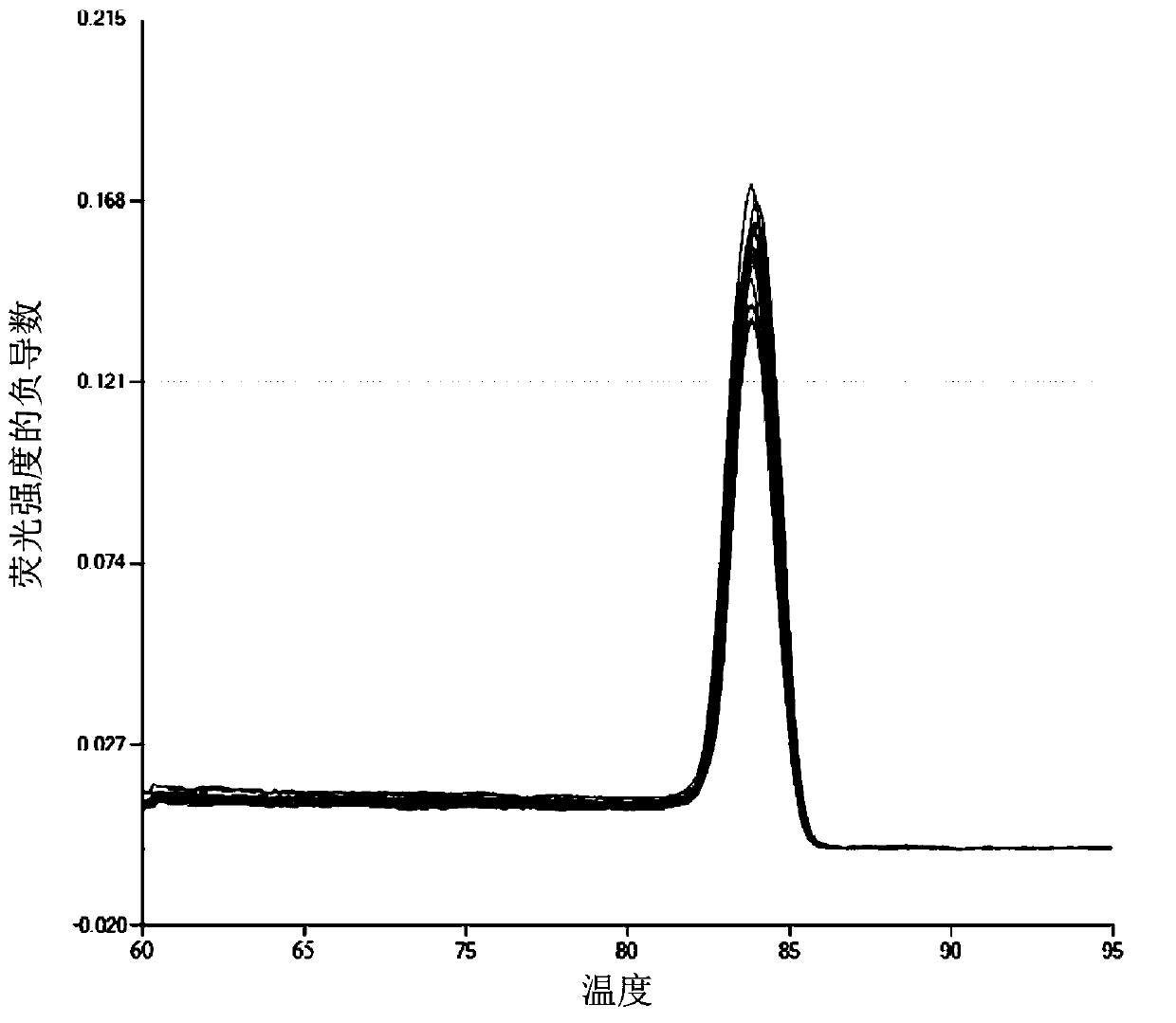
[0031] According to the experimental detection results, the melting curves of the quantitative PCR amplification using the vesicle virus-infected cell genome and the AV standard as templates are consistent, in...
Embodiment 2
[0032] The rapid determination of embodiment 2 vesicular virus HvAV-3h titer
[0033] 1. Virus collection and sample processing
[0034] Collect the hemolymph of beet armyworm infected with vesicle virus HvAV-3h strain 7 days in a 1.5ml centrifuge tube, sonicate (20w, 6min), and use Sf-900 II SFM medium for 10-fold serial dilution, and the dilution factor is (10 2 ~10 7 ), filtered through a 0.22 μm filter and stored at 4°C for later use.
[0035] 2. Cell Infection and Counting
[0036] Inoculate SeFB cells in a 6-well plate, and when the cells adhere to the wall and grow to 80-90% monolayer confluence, discard the supernatant and add 2 mL / well of the above virus dilution, with 3 replicates for each gradient concentration, and keep the temperature at 27°C Incubate for 1 hour, wash twice with PBS, collect the cells in a 1.5ml centrifuge tube, and count the number of virus cells with a hemocytometer.
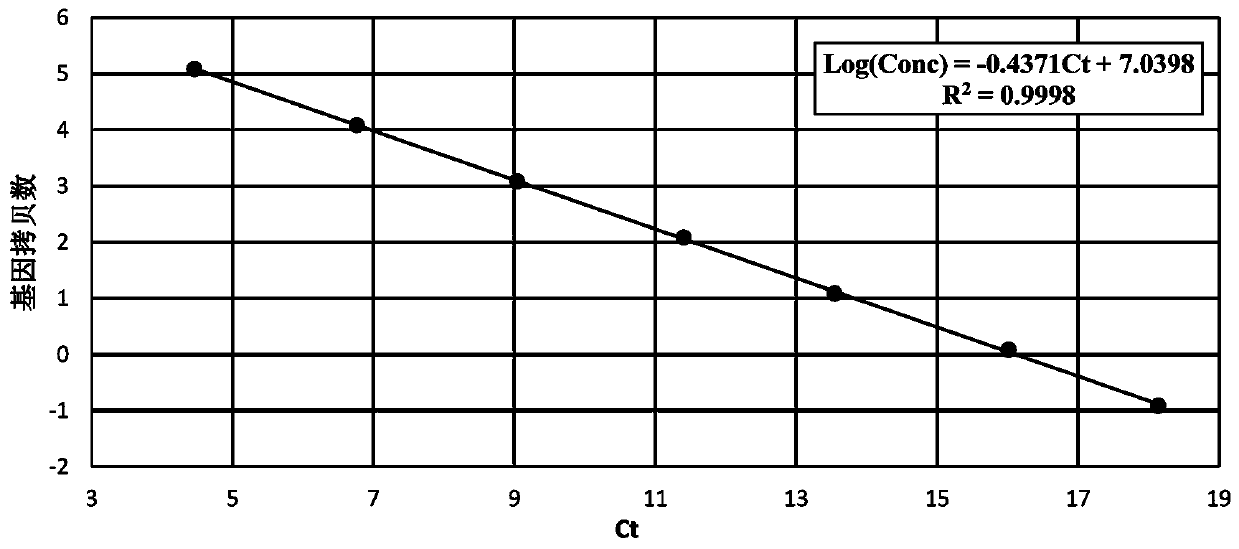
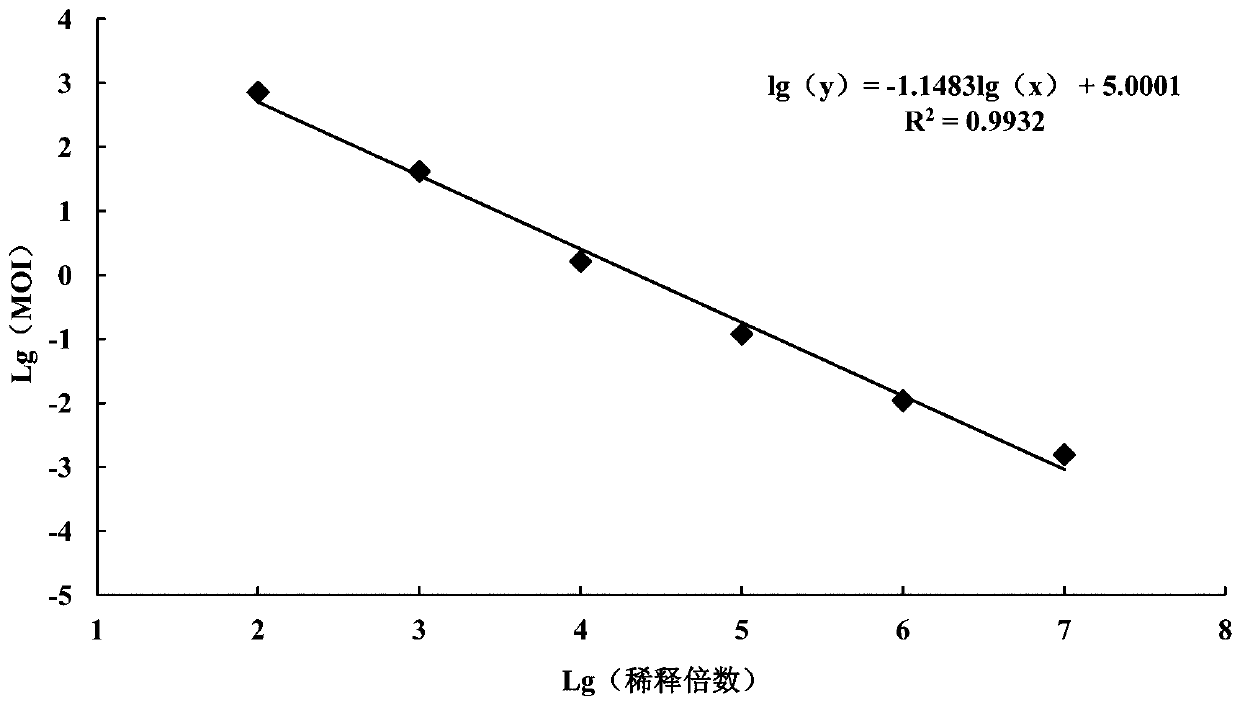
[0037] 3. Calculation of viral genome copy number
[0038]The collected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com