Solid pharmaceutical formulations for treating endometriosis, uterine fibroids, polycystic ovary syndrome and adenomyosis
A drug and solid technology, applied in the field of solid drug formulations for the treatment of endometriosis, uterine fibroids, polycystic ovary syndrome and adenomyosis, can solve problems such as adenomyosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
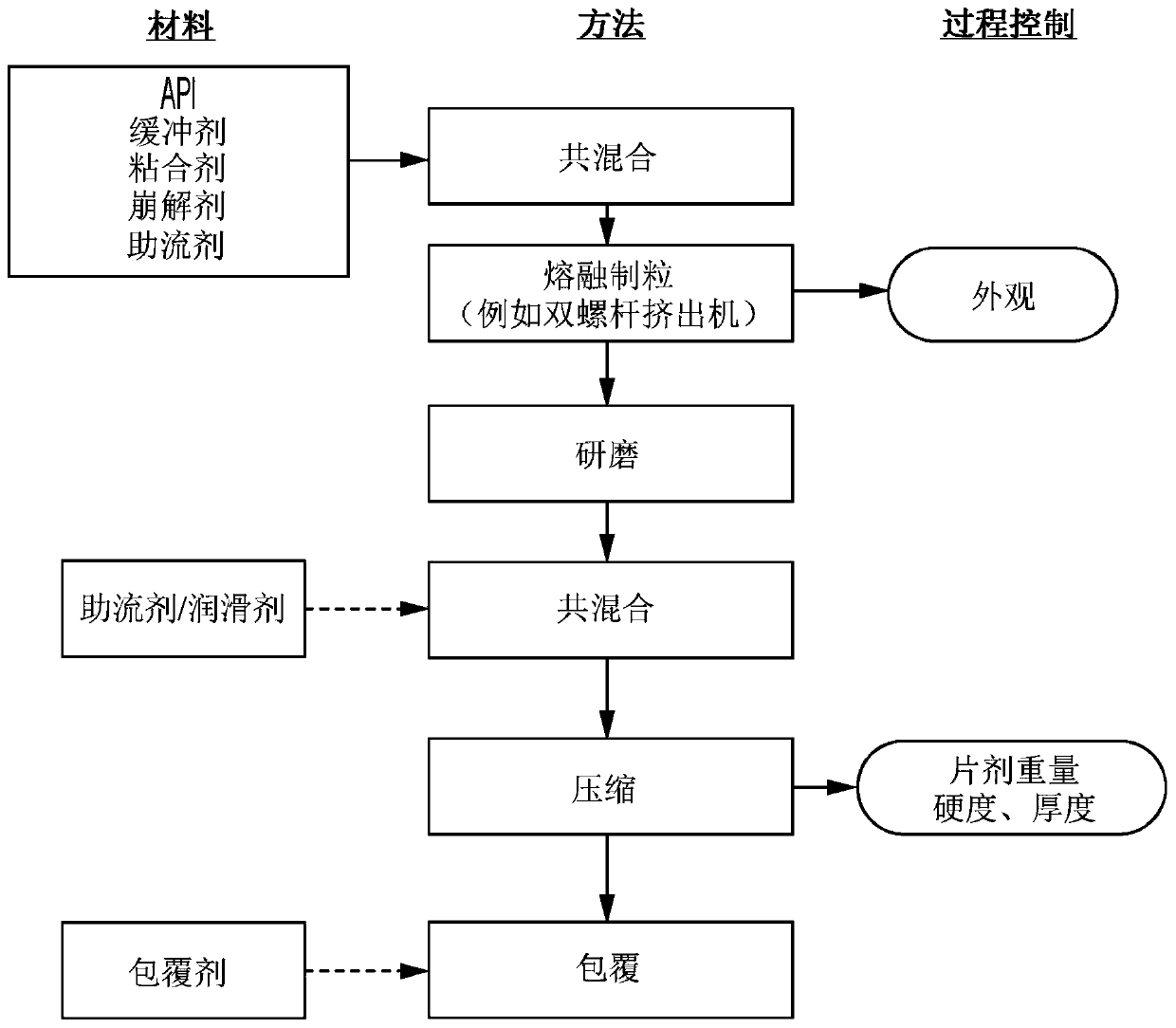
[0190] The preparation of the melt can be carried out in various ways. The mixing of the components can take place before, during or after the formation of the melt. For example, the components may be mixed and then melted, or mixed and melted simultaneously. The melt can also be homogenized in order to efficiently disperse the active ingredient. In addition, it may be expedient to melt the polymer first and then mix it into the active ingredient and homogenize it. Various additives may also be included in the melt, such as pH adjusters, glidants, disintegrants and / or fillers.
[0191] In certain embodiments, the solidified melt processed product is further ground, ground or otherwise reduced to particles. In some such embodiments, the melt processed product and each particle manufactured comprises a solid dispersion comprising the active ingredient and a pharmaceutically acceptable meltable binder. In some such embodiments, the melt processed product and each particle man...
example 1
[0212] Example 1: Formulations F1 and F2
[0213] Table 3 provides additional non-limiting examples of components of the disclosed formulations and the weight percent (w / w) of these components in the formulations. Compound A mentioned in the table below is the Compound A sodium salt and the corresponding weight percentages are provided in the salt form.
[0214] Table 3: Composition of formulations F1 and F2
[0215]
[0216] a Percentages given based on the weight of the uncoated tablet. Total percentages may not be 100% due to rounding.
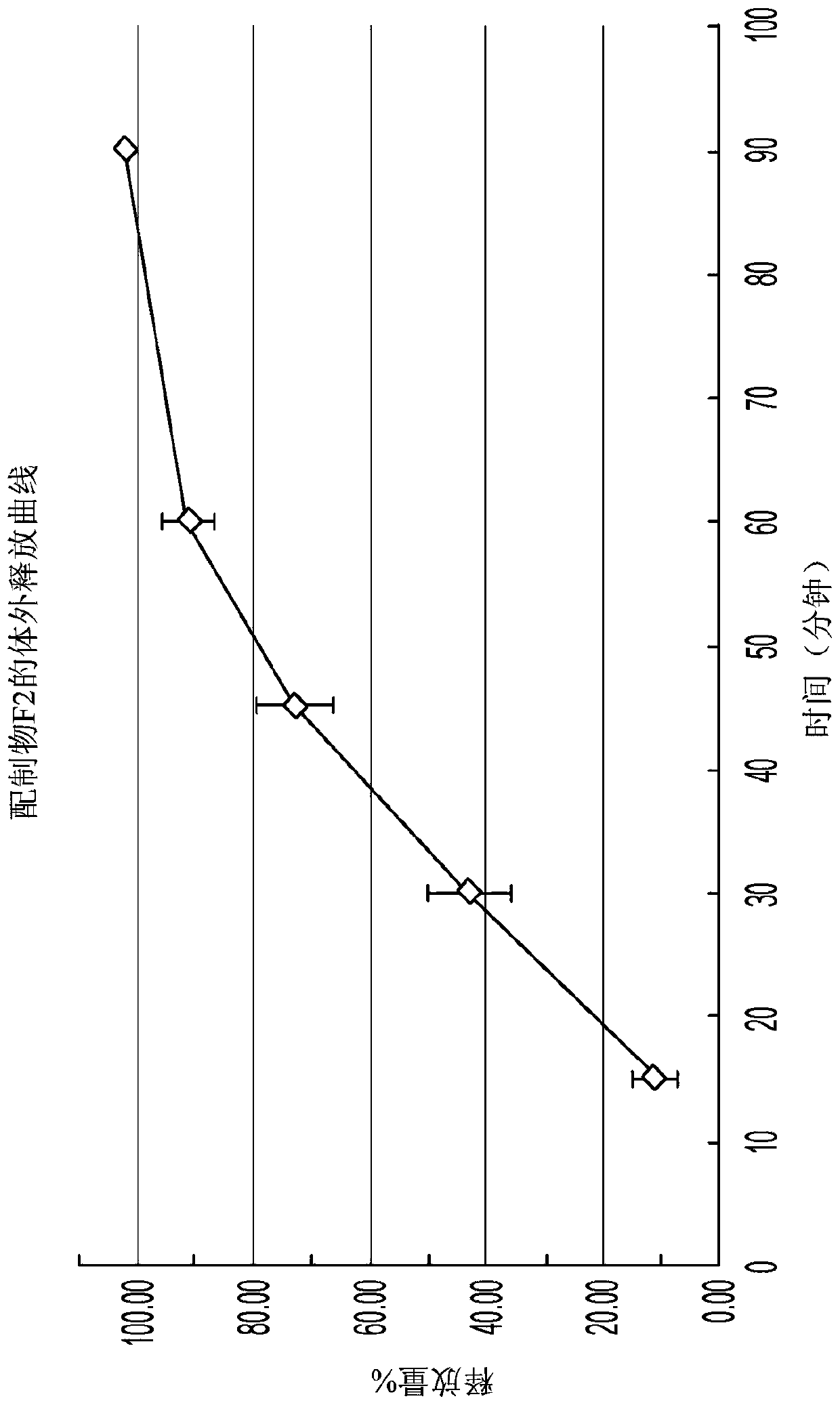
[0217] Formulation F2 was tested for dissolution in 900 mL of pH 6.8 sodium phosphate using USP Apparatus II at 37°C and 50 rpm paddle speed. The dissolution profile of the formulation F2 tablet is shown in figure 2 middle.
example 2
[0218] Example 2: Study on the effect of anti-gelling agents on chemical stability
[0219] In order to study the impact of anti-gelling agent (pH regulator) on the chemical stability of compound A in the formulation, the preparation containing different compound A and anti-gelling agent sodium carbonate ratio is as follows:
[0220] Table 4. Composition of Exemplary Formulations
[0221]
[0222] 95 g of compound A, 9.5 g of anhydrous sodium carbonate, 7.66 g of polyethylene glycol 3350 and 7.66 g of crospovidone were weighed and pre-blended. After sieving through a 30 mesh screen, 1.6 g of silicon dioxide was added to the powder mixture and blended for an additional 5 minutes. Using Thermo Scientific TMPharma 11 twin screw extruder, melt granulation of the powder blend at 200-600mg / hr and 123-124°C with a screw speed of 400rpm. Granules were obtained by milling the off-white opaque extrudate at 5000 rpm using a Fitzmill using positive impact and a 0.033" circular scree...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com