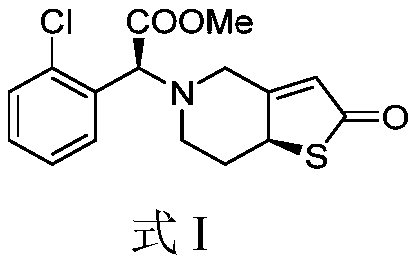
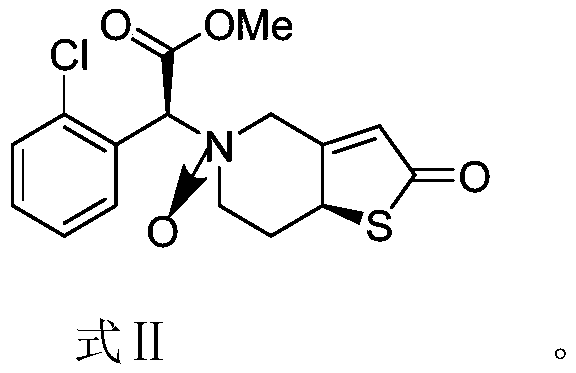
Solid preparation containing insoluble thienopyridine composition and preparation method
A technology for solid preparations and compositions, which is applied in the field of solid preparations and preparations containing insoluble thienopyridine compositions, can solve the problems of reduced bioavailability and slow dissolution speed, and achieve improved dissolution rate, low production cost, and preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment discloses the preparation method of the compound of formula II, specifically:
[0041] N-Oxo-(S)-2-(2-chlorophenyl)-2-((S)-2-oxo-2,6,7,7a-tetrahydrothiopheno[3,2-c]pyridine Synthesis of -5(4H)yl)methyl Acetate
[0042] Step 1. Resolution of the racemic product
[0043]
[0044] Referring to the patent CN104245707A, after we synthesized the structure of the SMX compound, the obtained racemic product 4.6gSMX was resolved by preparing a chiral column to obtain two relatively pure corresponding chiral isomers SMX-1 and SMX-2, 1.24g and 1.51g respectively. g. (Yield 59.8%) LC-MS (ESI) [M+H + ] + =338.8(M+H + ) is consistent with the structure.
[0045] The preparation of step 2 formula II compound:
[0046]
[0047] Put 1.51g of SMX-1 and 10mL of glacial acetic acid into a 50mL three-necked flask at room temperature, add 1ml of hydrogen peroxide dropwise in an ice bath, raise the temperature to 80°C for 2 hours after the addition, and monitor the...
Embodiment 2
[0048] Embodiment 2: the research of the different ratio of formula II compound and formula I compound
[0049] In this example, the pure products of the compound of formula I and compound of formula II in the thienopyridine composition with different proportions were added to conduct research to investigate their quality stability. Specifically: using the same amount of thienopyridine composition and auxiliary materials, only the ratio of the formula II compound to the formula I compound is different, and the same preparation method is used to make tablets. The content of the related substances was determined on the tablet at 0 days and accelerated at 6 months. Its prescription composition is calculated by 1000 tablets, as shown in Table 1:
[0050] Table 1 Prescription Composition Table 1
[0051]
[0052]
[0053] The preparation method is:
[0054]1. Weigh the pure products of the compound of formula I and the compound of formula II respectively; after mixing even...
Embodiment 3
[0066] Embodiment 3: control the ratio of formula II compound and formula I compound, the preparation method of refinement
[0067] According to the method disclosed in Example 2 of CN 104245707, prepare the compound crude product (containing II compound) synthesized and placed for a period of time shown in I, get 1.0g of the compound crude product shown in formula I and add in a 50mL eggplant-shaped bottle, add 30mL tetrahydrofuran, and heat up to Stir and dissolve at 60°C, basically dissolve, filter while hot, stir the filtrate in a 50mL beaker, and slowly cool down to room temperature, stir overnight to crystallize, and dry to obtain 0.50g of the finished compound. The mass ratio of the compound II to the compound of formula I in the finished product If it is less than or equal to 0.5:100, it can be refined repeatedly, so as to further reduce the mass ratio of the compound II to the compound of formula I.
[0068] The assay method of formula II compound and formula I compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com