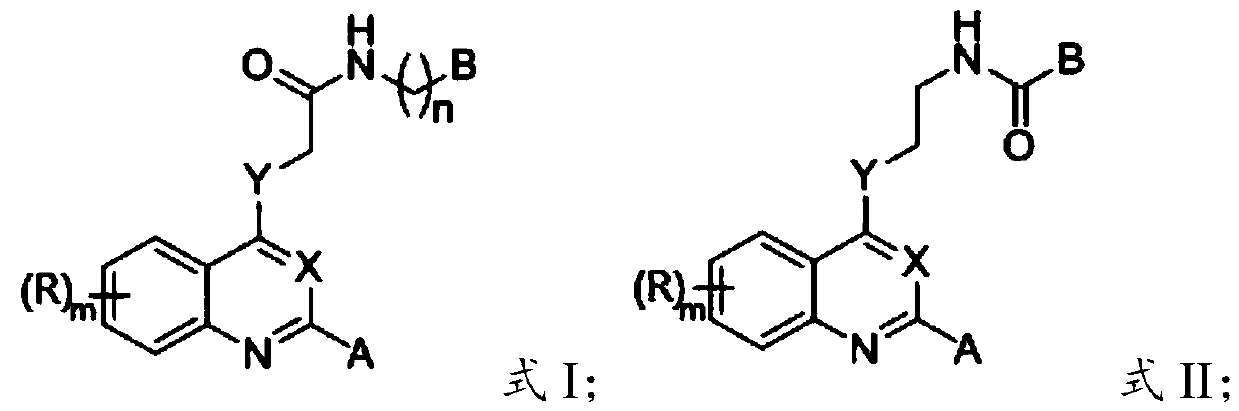
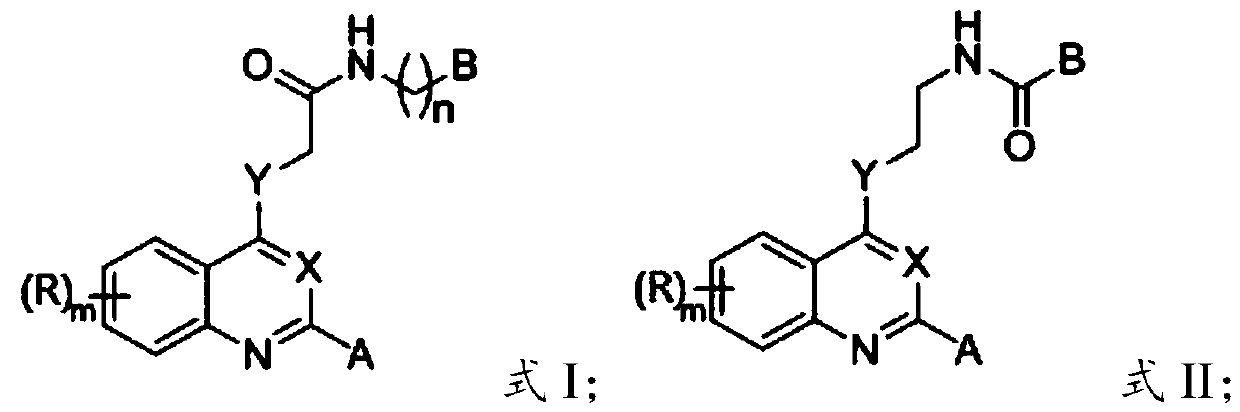
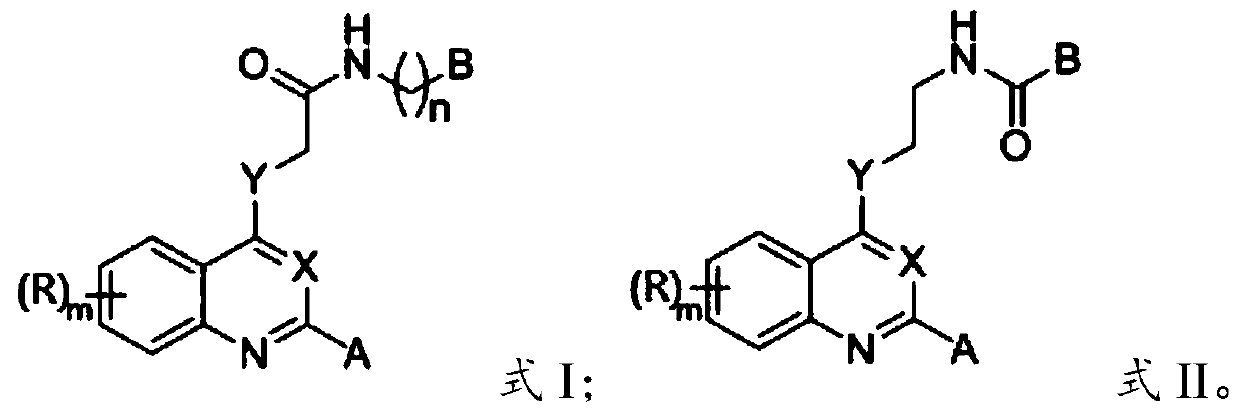
2-substituted-4-site functionalized N (O, S)-quinazoline derivative and application thereof
A technology of functionalization and derivatives, which is applied in the field of 2-substituted-4-functionalized N-quinoline derivatives to achieve good druggability, good application prospects, and obvious effects of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation of 2-((2-(pyrrolidin-1-yl) quinazoline-4-yl) amino)-N-phenylacetamide, the structural formula is shown in the following formula:
[0075]
[0076] 1) Preparation of N-tert-butoxycarbonylglycine
[0077] Dissolve glycine (30.84g, 410mmol) in 200mL of methanol, add triethylamine (180mL, 1230mmol), add di-tert-butyl dicarbonate (179.11g, 820mmol) in batches, gradually raise the temperature to 70°C and stir for 6 hours, start Gas is produced. TLC monitored whether the reaction of the raw material was complete, concentrated under reduced pressure, added 200 mL of ethyl acetate, washed twice with water, washed once with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 36.41 g of a light yellow solid, which was directly obtained without further purification. for the next step. ESI-MS, m / z: 174.3 [M-H] - .
[0078] 2) Preparation of tert-butoxy (2-oxo-2-anilinoethyl) carbamate
[0079] N...
Embodiment 2
[0087] The preparation of 2-((2-pyrrolidinylquinazolin-4-yl)amino)-N-(β-naphthyl)acetamide, the structural formula is as follows:
[0088]
[0089] 1) Preparation of tert-butoxy (2-oxo-2-(β-naphthylamino) ethyl) carbamate
[0090] In Example 1, 2) aniline (2.43g, 26.1mmol) in the preparation of tert-butoxy (2-oxo-2-anilinoethyl) carbamate was replaced by β-naphthylamine (3.74g, 26.1 mmol) and other operations unchanged, tert-butoxy (2-oxo-2-(β-naphthylamino) ethyl) carbamate was obtained.
[0091] ESI-MS, m / z: 301.4 [M+H] + .
[0092] 2) Preparation of 2-amino-N-(β-naphthyl)acetamide hydrochloride
[0093] In Example 1, 3) tert-butoxy (2-oxo-2-anilinoethyl) carbamate (4.50 g, 18 mmol) was replaced by tert-butoxy (2-oxo-2-(β -Naphthylamino)ethyl)carbamate, and the other operations were unchanged to obtain 2-amino-N-(β-naphthyl)acetamide hydrochloride as a pale yellow solid. ESI-MS, m / z: 151.3 [M+H] + .
[0094] 3) Preparation of 2-((2-chloroquinazolin-4-yl)amino)-N-(β...
Embodiment 3
[0099] The preparation of 2-((2-(pyrrolidin-1-yl) quinazoline-4-yl) oxy)-N-phenylacetamide, the structural formula is shown in the following formula:
[0100]
[0101] 1) Preparation of 2-((2-chloroquinazolin-4-yl)oxy)ethyl acetate
[0102] Under ice-cooling, dissolve 60% sodium hydride (0.50 g, 12 mmol) in 15 mL of N,N-dimethylformamide, add ethyl glycolate (1.07 g, 10 mmol), stir at the same temperature for 10 minutes, then add 2, 4-Dichloroquinazoline (2.04 g, 10 mmol), stirred at the same temperature for 20 minutes and then at room temperature overnight. TLC detects that the raw materials have reacted completely, add 30 mL of water to the reaction solution, extract with ethyl acetate (20 mL×2), combine the organic phases, wash with deionized water and saturated sodium chloride solution successively, concentrate, silica gel column chromatography, petroleum ether / ethyl acetate 15:1 eluted to obtain 2.04 g of off-white solid. 1 H NMR (500MHz, DMSO-d 6 )δ8.26–8.15(m,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com