Preparation process of bivalirudin for injection
A bivalirudin and preparation technology technology, applied in the field of pharmaceutical preparations, can solve the problems of bivalirudin precipitation and dispensing time, etc., and achieve the effects of shortening the dispensing time, reducing microbial load, and reducing related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation process of bivalirudin for injection, comprising the following steps:
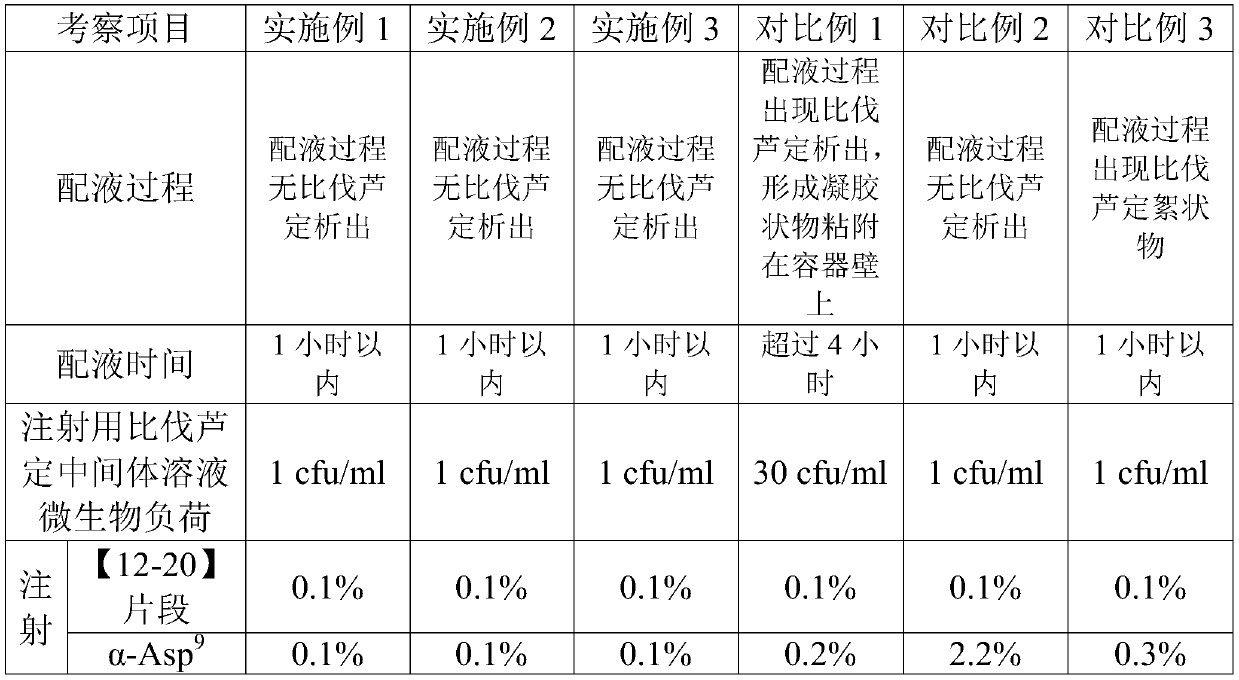
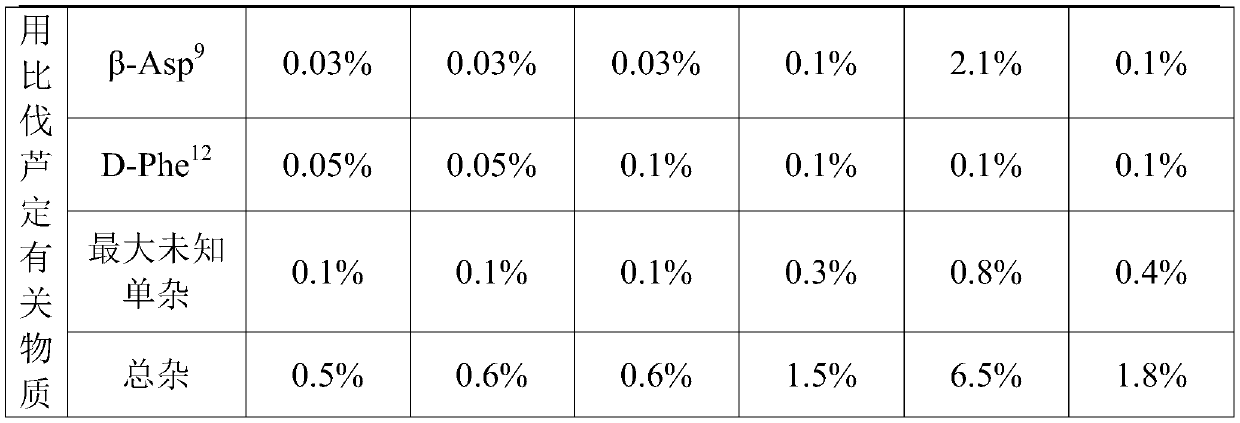
[0026] (1) Take 1.25 kg of mannitol, dissolve it in 28.5 L of water for injection, stir to dissolve, then add 2.5 kg of bivalirudin (in dry form), and stir to dissolve to obtain a mixed solution of bivalirudin and mannitol;
[0027] (2) Bivalirudin mannitol mixed solution and 0.5mol / L sodium hydroxide solution are simultaneously pumped into the liquid mixing tank through different pipelines, stirred and mixed, and the flow rate of the bivalirudin mannitol mixed solution is: 720ml / min, the flow rate of the sodium hydroxide solution is 240ml / min, that is, the flow ratio of the two is 3:1, keep stirring, the stirring rate is 300rpm~400rpm, adjust the pH value of the solution to 5.0~6.0, add water for injection to 50L, to obtain bivalirudin intermediate solution for injection; the temperature of the whole solution is 10℃~20℃;
[0028] (3) The bivalirudin intermediate solution for injectio...
Embodiment 2
[0030] A preparation process of bivalirudin for injection, comprising the following steps:
[0031] (1) Take 1.25kg of mannitol, dissolve it in 19L of water for injection, stir to dissolve, then add 2.5kg of bivalirudin (in dry form), stir to dissolve, and obtain a mixed solution of bivalirudin and mannitol;
[0032] (2) Bivalirudin mannitol mixed solution and 0.25mol / L sodium hydroxide solution are pumped into the liquid mixing tank through different pipelines simultaneously, stirred and mixed, and the flow rate of described bivalirudin mannitol mixed solution is: 600ml / min, the flow rate of the sodium hydroxide solution is 600ml / min, that is, the flow ratio of the two is 1:1, keep stirring, the stirring rate is 150rpm-200rpm, adjust the pH value of the solution to 5.0-6.0, add water for injection to 50L, to obtain the bivalirudin intermediate solution for injection; the whole dosing temperature is 5°C to 10°C;
[0033] (3) The bivalirudin intermediate solution for injection...
Embodiment 3
[0035] A preparation process of bivalirudin for injection, comprising the following steps:
[0036] (1) Take 1.25 kg of mannitol, dissolve it in 28.5 L of water for injection, stir to dissolve, then add 2.5 kg of bivalirudin (in dry form), and stir to dissolve to obtain a mixed solution of bivalirudin and mannitol;
[0037] (2) Bivalirudin mannitol mixed solution and 1.5mol / L sodium hydroxide solution are pumped into the liquid mixing tank through different pipelines simultaneously, stirred and mixed, and the flow rate of described bivalirudin mannitol mixed solution is: 900ml / min, the flow rate of the sodium hydroxide solution is 100ml / min, that is, the flow ratio of the two is 9:1, keep stirring, the stirring rate is 400rpm-500rpm, adjust the pH value of the solution to 5.0-6.0, add water for injection to 50L, to obtain the bivalirudin intermediate solution for injection; the whole dosing temperature is 20°C to 25°C;
[0038] (3) The bivalirudin intermediate solution for in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com