Preparation method of 1,3-cyclohexanedione
A technology of cyclohexanedione and resorcinol, which is applied in the field of recovery of high-purity 1,3-cyclohexanedione preparation, can solve problems such as unsolved problems, low product yield, and 1,3-cyclohexanedione efficiency. and low recovery rate, to achieve the effect of easy operation and low organic matter content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
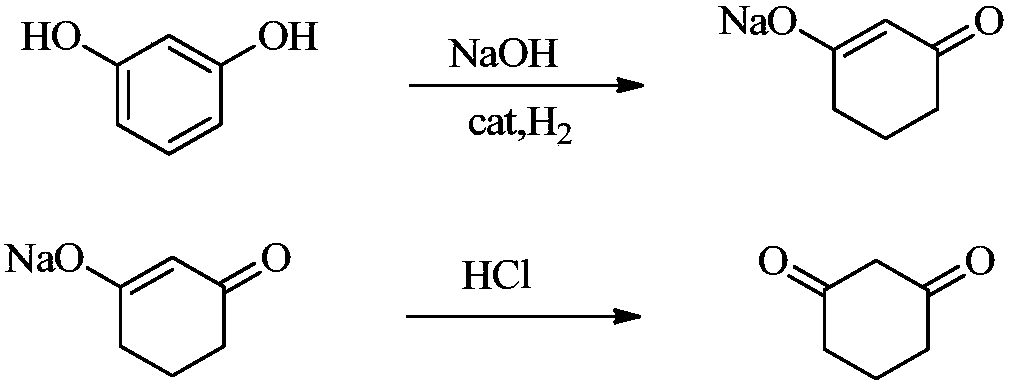
[0036] The preparation method that the present invention provides 1,3-cyclohexanedione comprises the steps:
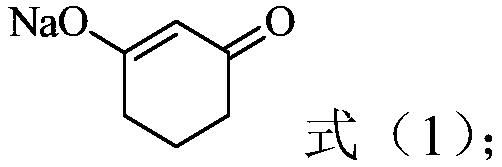
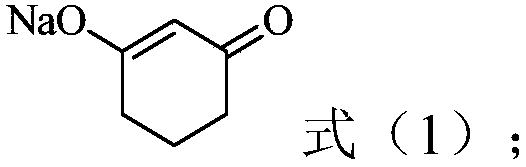
[0037] (1) In the presence of water, resorcinol is hydrogenated to obtain a reaction solution containing a compound represented by the following formula (1), and the reaction solution is acidified to obtain 1,3-cyclohexanedione acidified solution, Then crystallize the 1,3-cyclohexanedione acidified solution to obtain 1,3-cyclohexanedione product and acidified mother liquor containing 1,3-cyclohexanedione,
[0038]
[0039] (2) In the presence of an organic solvent, the acidified mother liquor containing 1,3-cyclohexanedione is contacted with a complexing agent, so that 1,3-cyclohexanedione is complexed with the complexing agent, and Obtain the organic phase;
[0040] (3) contacting the organic phase obtained in step (2) with the reaction solution, and adjusting the pH to 7-14, so that 1,3-cyclohexanedione and the complexing agent are dissociated, and an aqueous pha...
Embodiment 1
[0069] (1) hydrogenation, acidification and crystallization
[0070] Resorcinol (99%, 0.535mol) is neutralized with aqueous sodium hydroxide solution (15%, 0.589mol), and then hydrogenated at 5MPa and 120°C to obtain the formula The reaction solution of the compound shown (30%, 0.535mol), and the reaction solution was acidified with hydrochloric acid (30%, 0.562mol) at 15-20°C to obtain 1,3-cyclohexanedione acidified solution , and then cool down the 1,3-cyclohexanedione acidification solution to crystallize at 5°C and filter and dry to obtain 1,3-cyclohexanedione product (99%, 0.426mol) and 1,3-cyclohexanedione acidified mother liquor.
[0071] (2) Extraction of 1,3-cyclohexanedione
[0072] The first extraction: at room temperature, add 75g of trioctylamine (i.e. tri-n-octylamine) and 125g of kerosene to a 1L reaction bottle to obtain a mixed solution of complexing agent, and add the 1 obtained in step (1) under stirring. The acidified mother liquor of 3-cyclohexanedione...
Embodiment 2
[0080] Prepare 1,3-cyclohexanedione according to the method of Example 1, the difference is that in step (2), the first extraction uses the upper trioctylamine kerosene phase extracted for the second time in Example 1, and the second extraction The upper trioctylamine kerosene phase extracted for the third time in Example 1 was used for extraction.
[0081] The 1,3-cyclohexanedione product (99.4%, 0.519 mol) was finally obtained with a yield of 96.43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com