Biodegradable microparticles for sustained delivery of Anti-angiogenic peptide
An anti-angiogenesis, microparticle technology, applied in the direction of cardiovascular system diseases, peptides, drug combinations, etc., can solve the problems of unfavorable patient compliance, discomfort, and low frequency of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
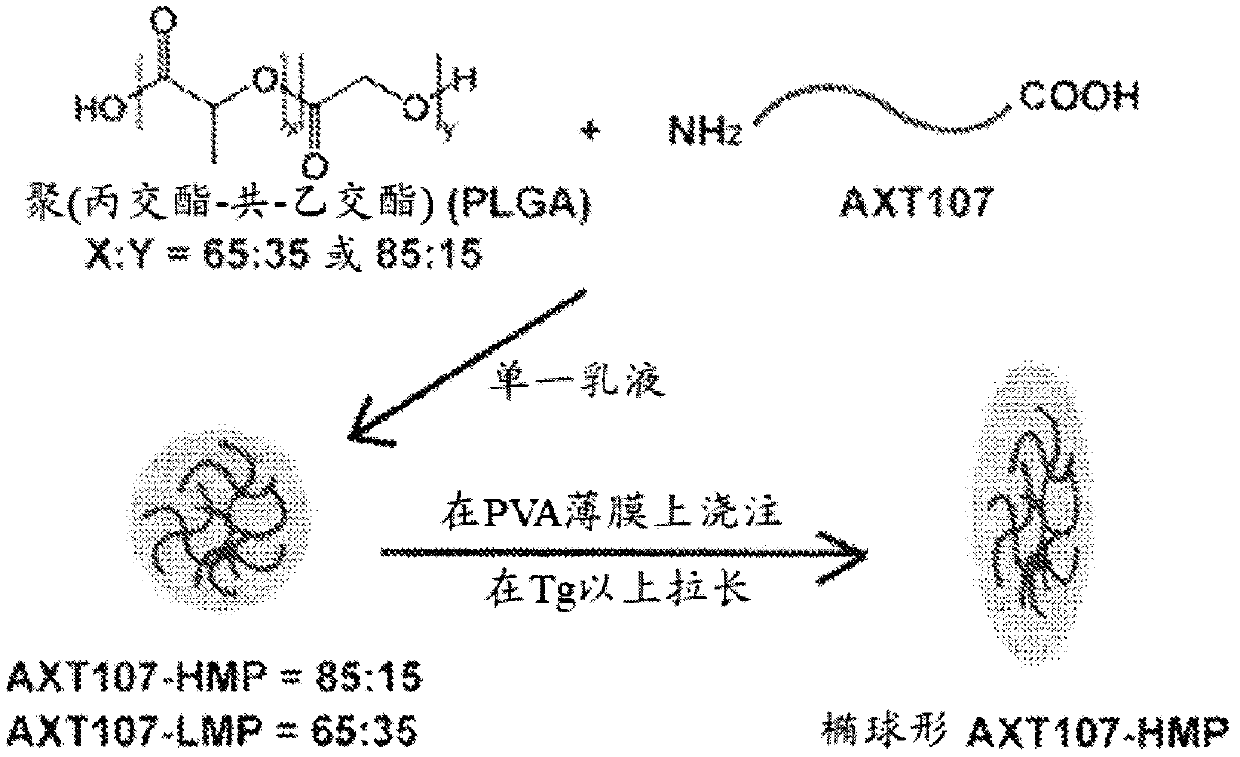
[0097] Example 1: Microparticles with Higher Lactic to Glycolic Acid Ratio Have Longer Peptide Release Relative to Microparticles with Lower Lactic to Glycolic Acid Ratio
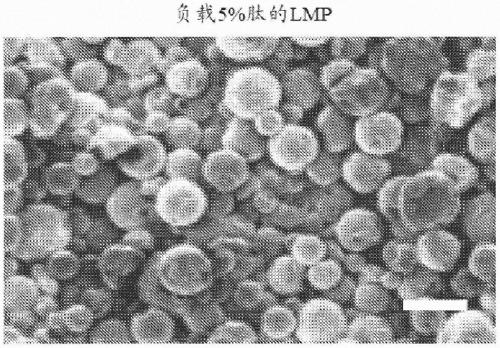
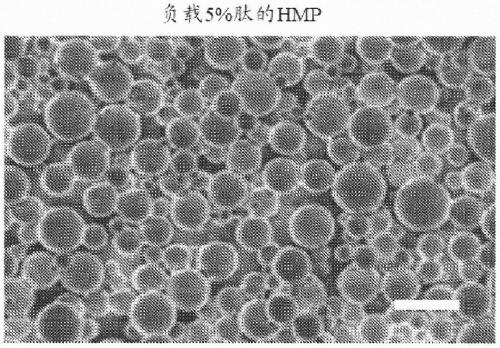
[0098] Preparation of poly(lactide-co-glycolide) containing poly(lactide-co-glycolide) with various ratios of lactic acid (LA) to glycolic acid (GA) (LA:GA ratio) and loaded with AXT107 peptide (which has the amino acid sequence of SEQ ID NO:3) ester) "PLGA" microparticles. As an example, low lactic acid microparticles (LMP) comprising a 65:35 LA:GA ratio, medium lactic acid microparticles (MMP) comprising a 75:25 LA:GA ratio, and 85:15 LA:GA ratio were evaluated in this example. High Lactic Acid Microparticles (HMP) with a GA ratio; see figure 1 . When not elongated at a temperature above its polymer transition temperature (see figure 1 ), LMP and HMP loaded with 5% peptide were found to be roughly spherical (see Figure 2A and Figure 2B ), and has an average diameter of about 5 microns (see Figure...
Embodiment 2
[0102] Example 2: AXT107-containing microparticles reduce neovascularization and vascular leakage in vivo
[0103] The ability of AXT107-containing microparticles to treat and / or alleviate symptoms of choroidal neovascularization (CNV) and subretinal neovascularization (subretinal NV) was evaluated. CNV and subretinal NV are associated with age-related macular degeneration (AMD), and particularly "wet" AMD. Here, basically image 3 Mouse models of CNV or subretinal NV were evaluated as shown in .
[0104] Such as Figure 4A As shown in , AXT107-containing microparticles were as effective as aflibercept, an FDA-approved therapeutic agent for wet macular degeneration, in inhibiting laser-induced CNV; A). Notably, the combination of aflibercept and AXT107-containing microparticles (A+AXT107) showed additional inhibition over either treatment alone. Figure 4B AXT107-containing microparticles were shown to promote CNV regression in a dose-dependent manner.
[0105] Similarly,...
Embodiment 3
[0108] Example 3: AXT107-containing HMPs provide longer-lasting in vivo effectiveness than AXT107-containing LMPs
[0109] Such as Figure 5A As shown in, AXT107-containing HMPs provide long-term (at least until 16 weeks post-injection) in vivo efficacy in inhibiting laser-induced CNV; they promote in vivo regression of CNV following laser induction; see Figure 5B . Such as Figure 5C As shown in , AXT107-containing LMPs provided prolonged (at least until 8 weeks post-injection) in vivo efficacy in inhibiting laser-induced CNV. At 12 weeks post-injection, there appeared to be little difference between LMPs containing AXT107 and empty LMPs. Like AXT107-containing HMPs, AXT107-containing LMPs promote regression in vivo following laser induction; see Figure 5D .
[0110] Finally, AXT107-Containing HMPs Effectively Treat Vascular Leakage in Transgenic rhoVEGF Mice, a Mouse Model of Subretinal NV; see Figure 5E and Figure 5F .
[0111] These data suggest that microparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com