A kind of nitrilase mutant with improved reaction specificity and application thereof
A nitrilase and mutant technology, applied in the field of enzyme engineering, can solve the problems that mutants cannot be applied to industrial biocatalytic processes, nitrile hydration activity enhancement or incomplete elimination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Selection of nitrilase mutation sites
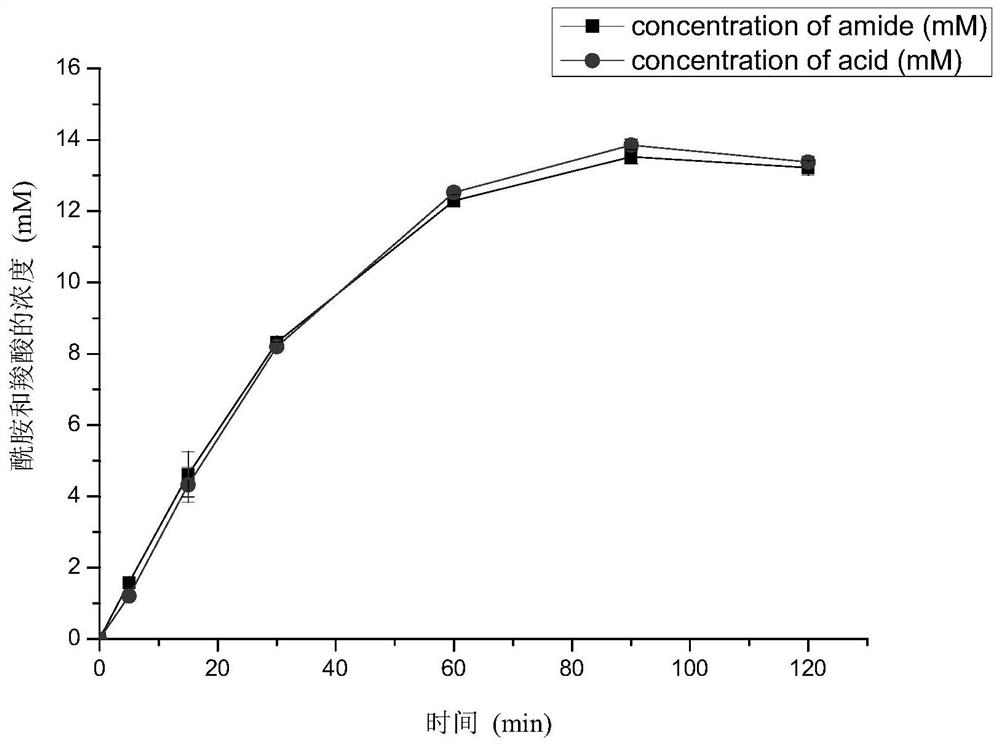
[0034]When rice nitrilase (OsNIT, GenBank accession number: AB027054, amino acid sequence SEQ ID NO.1, nucleotide sequence SEQ ID NO.2) catalyzes the reaction of benzyl nitrile, the ratio of amide and carboxylic acid in the product is close to 1:1, Such as figure 1 shown. Through bioinformatics analysis, the present invention determines that its catalytic triplet is 196Cys-71Glu-162Lys, and further screens out 18 amino acid residues near the catalytic pocket that may affect the specificity of the reaction: A72, A167, F175, E198, Y77, S229, T166, E169, I195, R224, S230, W197, H159, N199, K200, T220, A221 and V226.
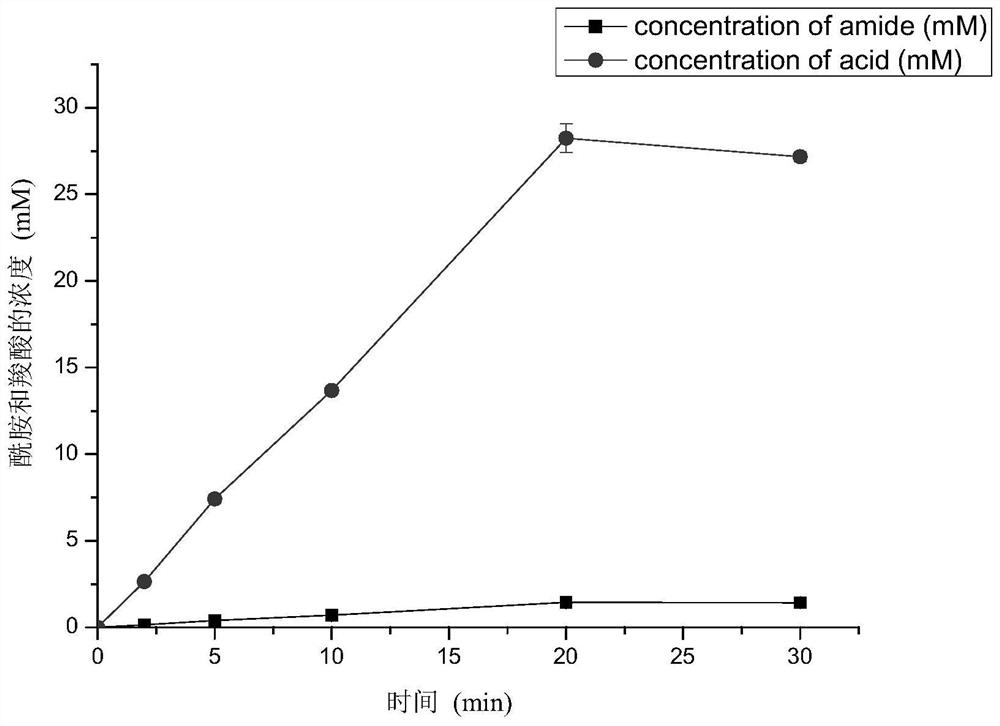
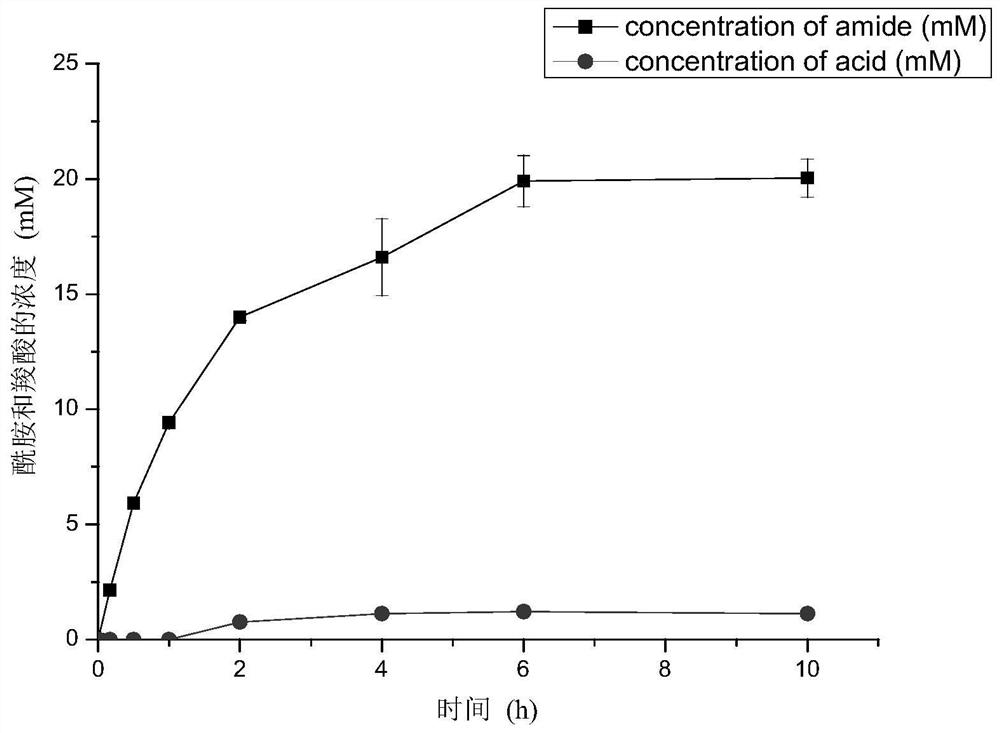
[0035] By performing site-directed saturation mutations on the above amino acid residues, six mutation sites Y77, I195, W197, N199, K200, T220, R224 and V226 that have a greater impact on the specificity of the catalytic reaction were obtained, including Y77, K200 , R224 and V226 site mutations of the nitrilase mu...
Embodiment 2
[0057] 1. Construction and expression of nitrilase multiple mutants
[0058] Using the mutant plasmid with excellent reaction specificity or high enzyme activity obtained in Example 1 as a template, combined with excellent mutations at other sites, such as the PCR system in Example 1, amplifying the entire plasmid by PCR to obtain multiple mutations The mutants were transformed by heat shock, and the mutants were introduced into E.coli BL21 (DE3) competent cells to construct multiple mutant engineering bacteria, and the constructed multiple mutants were expressed.
[0059] 2. Determination of the product ratio and activity of wild-type strains and multiple mutants containing nitrilase
[0060] Vitality determination was carried out on the obtained nitrilase multiple mutant strains. The composition of the reaction system: 10 mL of Tris-HCl buffer solution (50 mM, pH 8.0), 10 g / L of wet bacteria, and 30 mM of phenylacetonitrile (dissolved in methanol). The reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com