Nitrilase mutant, engineering bacterium and application of nitrilase mutant
A technology of nitrilase and mutant, applied in the directions of hydrolase, application, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The construction of embodiment 1 nitrilase mutant
[0024] 1. Construction of mutants
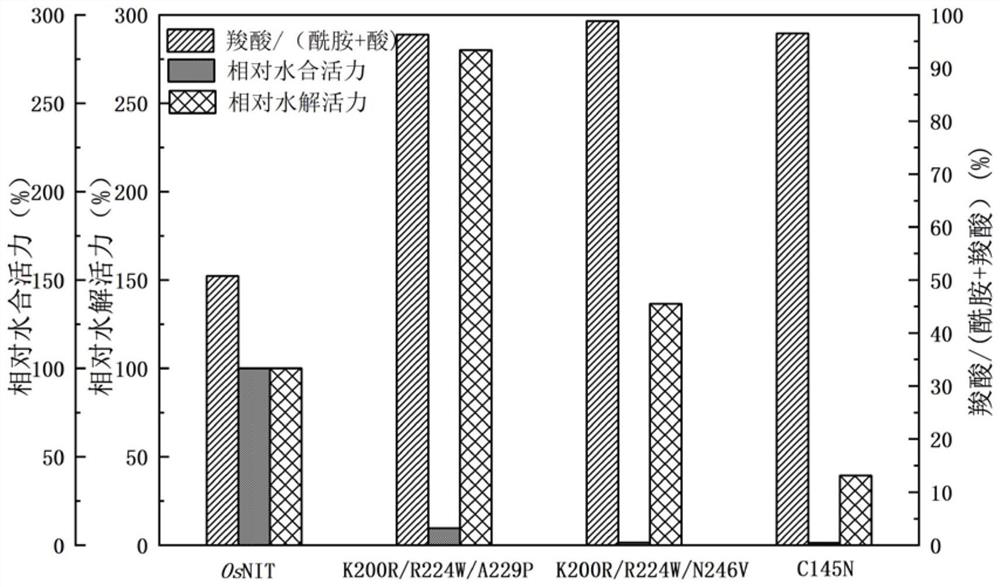
[0025] When rice nitrilase (OsNIT, GenBank accession number: AB027054, amino acid sequence SEQ ID NO.1) catalyzes the reaction of phenylacetonitrile, the ratio of amide to carboxylic acid in the product is close to 1:1. Through bioinformatics analysis, it was determined that its catalytic triplet was 196Cys-71Glu-162Lys, and then through rational design analysis, the 145th cysteine, the 200th lysine, the 224th arginine, and the 229th alanine Amino acid and asparagine at position 246 were subjected to site-directed saturation mutation, and a mutant with significantly improved reaction specificity was constructed, specifically:
[0026] The rice nitrilase (OsNIT, GenBank accession number: AB027054) gene (amino acid sequence SEQ ID NO.1, nucleotide sequence is SEQ ID NO.2) was inserted between the pET-28b vector BamH I and Hind III sites to construct recombinant plasmid. Using the re...
Embodiment 2
[0050] Example 2 Nitrilase Mutant C145N, K200R / R224W, A229P, N246V Reaction Specificity and Activity Determination
[0051] Using the plasmid of the K200R mutant obtained in Example 1 as a template, R224W site-directed mutagenesis primers (Table 1 Lys200-For, Lys200-Rev) were used for PCR amplification of the whole plasmid. The PCR system and reaction conditions were the same as in step 1, and the method in Example 1 was used. A plasmid of the mutant K200R / R224W (the amino acid sequence is shown in SEQ ID NO.3) was constructed, and wet cells were prepared according to the method in Example 1.
[0052] The reaction system (20mL) consists of: 20mL Tris-HCl buffer solution (50mM, pH 8.0), 0.1g of wet thallus of the nitrilase mutant prepared by the method of Example 1, 0.9mL of methanol, 100mM of phenylacetonitrile (benzylnitrile first soluble in methanol). The reaction solution was reacted at 30° C., 180 rpm for 30 minutes. Take 1 mL of sample, add 20 μL of 2M HCl to terminate ...
Embodiment 3
[0057] Example 3 Construction of nitrilase multiple mutant K200R / R224W / A229P.
[0058] The results of the single point mutation in Example 1 were analyzed, and it was found that the 200th, 224th, and 229th point mutations can change the specificity of the reaction so that it tends to generate carboxylic acid, and the hydrolysis activity is better retained or improved. The hydration activity was inhibited, and the mutant K200R / R224W / A229P was obtained by superposition mutation, specifically:
[0059] Using the K200R / R224W mutant plasmid constructed in Example 2 as a template, the primers at position 229 (Table 1Ala229-For, Ala229-Rev) were used for PCR amplification of the whole plasmid. The PCR system and reaction conditions were the same as in step 1, and Example 1 was used. Methods The plasmid containing the nitrilase mutant K200R / R224W / A229P was constructed.
[0060] Cultivate and obtain wet thalline cells capable of measuring carboxylic acid ratio and activity by the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com