Cell membrane fluorescent probe with high brightness, high stability and insensitivity to environment
A high-stability, fluorescent probe technology, applied in the field of fluorescence imaging, can solve the problems of poor photostability, long staining time, weak fluorescence, etc., and achieve high-stability results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
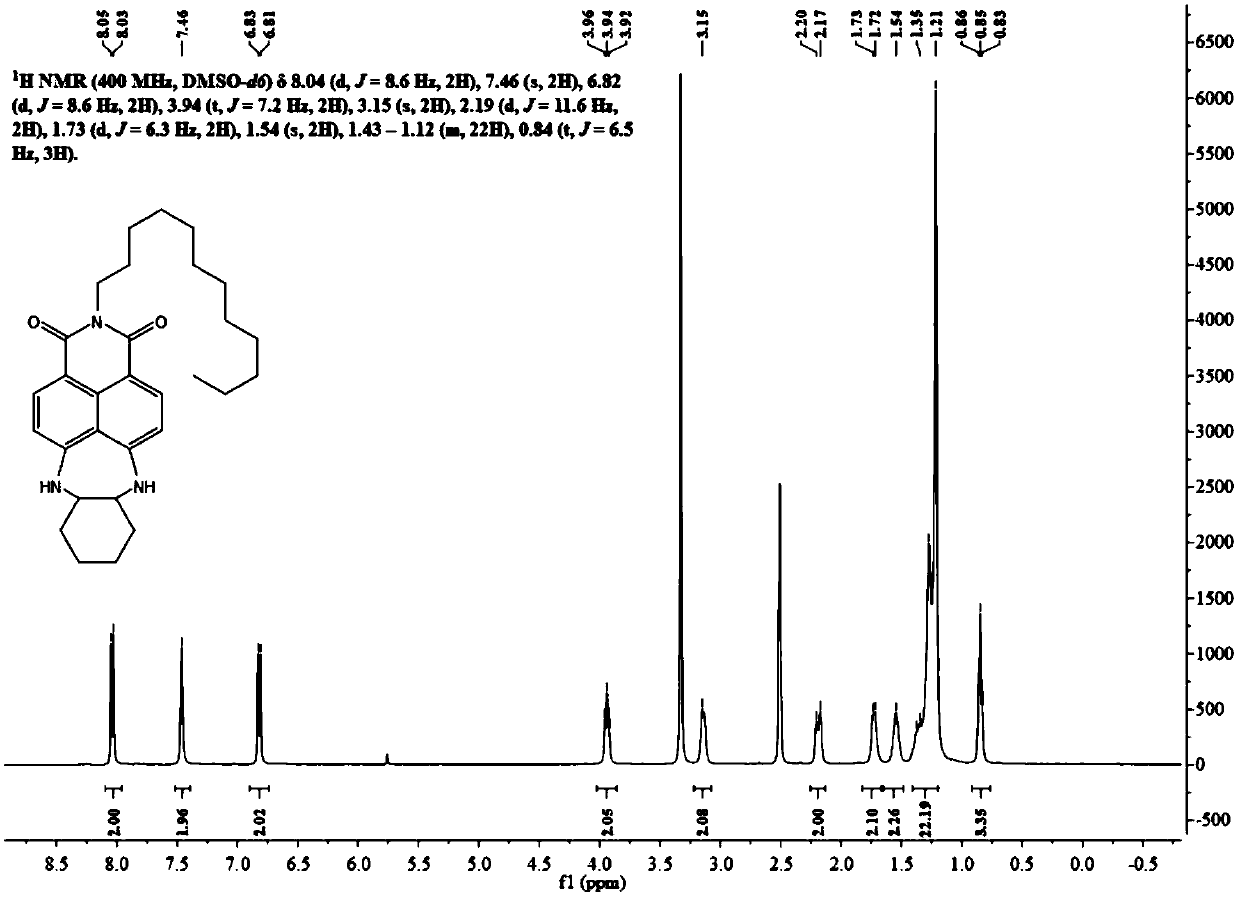
[0067] Synthesis of cell membrane probe DDAN-DAC.
[0068] Synthesis of intermediate DDAN-NBr:
[0069]
[0070] 4-Bromo-5-nitro-1,8-naphthalimide (0.50g, 1.56mmol) was dissolved in 30mL of ethanol, and dodecylamine (0.87g, 4.68mmol) was added dropwise thereto, heated to 90 After reacting at ℃ for 24 h, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether / dichloromethane=2 / 1, V / V) to obtain 0.54 g of DDAN-NBr as a yellow-white solid with a yield of 71%.
[0071] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0072] 1 H NMR (400MHz, CDCl 3 )δ8.71(d, J=7.9Hz, 1H), 8.51(d, J=7.8Hz, 1H), 8.23(d, J=7.8Hz, 1H), 7.94(d, J=7.8Hz, 1H) ,3.66(t,J=6.4Hz,2H),1.1-1.8(m,20H),0.94(t,J=7.9Hz,3H).
[0073] After detection, its structure is shown in the above formula DDAN-NBr.
[0074] Synthesis of cell membrane probe DDAN-DAC:
[0075]
[0076] DDAN-NBr (0.25g, 0.51mmol) was dissolve...
Embodiment 2
[0083] Synthesis of cell membrane probe HexAN-DAC.
[0084] Synthesis of intermediate HexAN-NBr:
[0085]
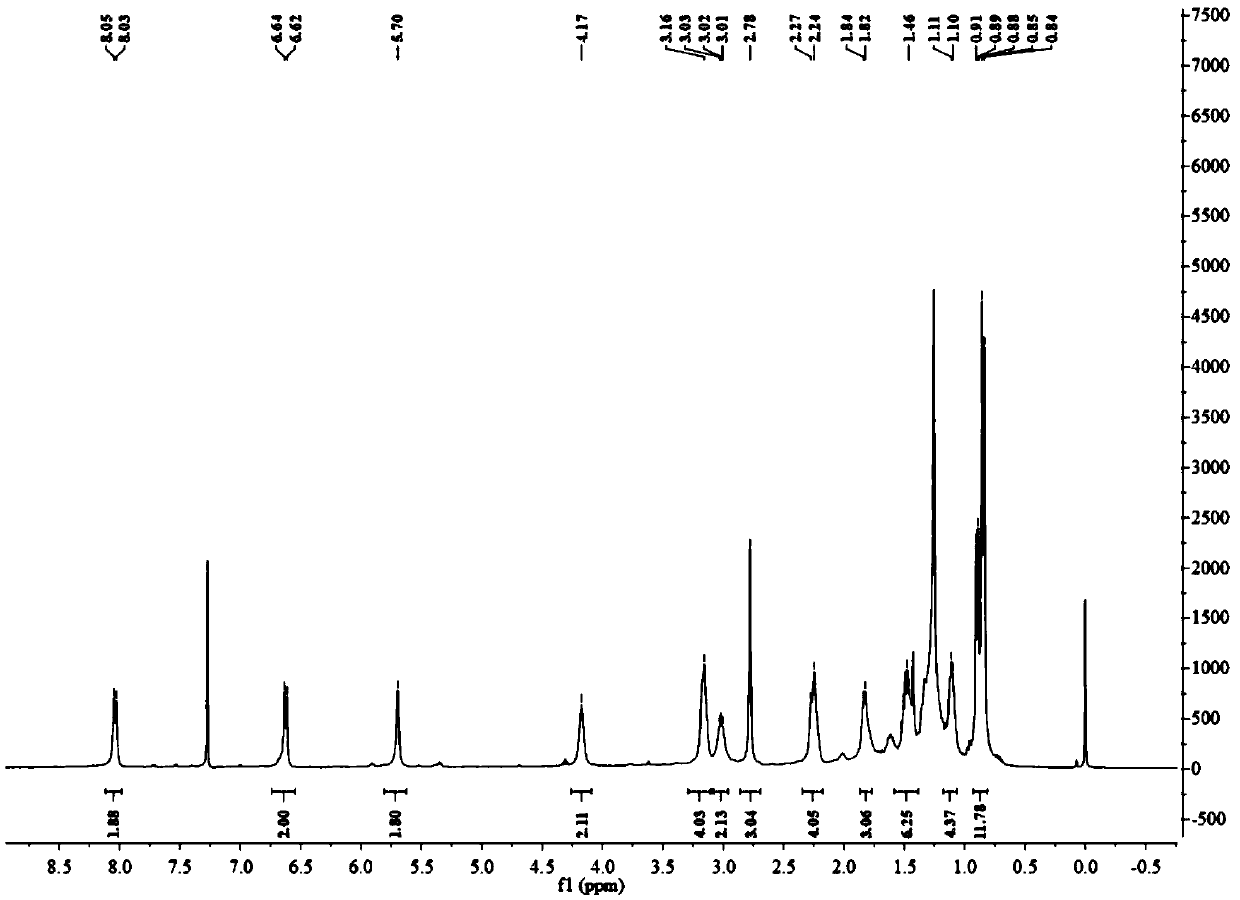
[0086] Dissolve 4-bromo-5-nitro-1,8-naphthalimide (0.50g, 1.56mmol) in 60mL of ethanol, and add hexadecylamine (0.11g, 4.68mmol) to it, and heat to 90°C After reacting for 24 hours, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether / dichloromethane=2 / 1, V / V) to obtain 0.52 g of a yellow-white solid HexAN-NBr, with a yield of 62%.
[0087] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0088] 1 H NMR (400MHz, CDCl 3)δ8.71(d, J=7.7Hz, 1H), 8.51(d, J=7.9Hz, 1H), 8.23(d, J=7.8Hz, 1H), 7.94(d, J=7.9Hz, 1H) ,3.63(t,J=6.5Hz,2H),1.1-1.8(m,28H),0.92(t,J=7.8Hz,3H).
[0089] After detection, its structure is shown in the above formula HexAN-NBr.
[0090] Synthesis of cell membrane probe HexAN-DAC:
[0091]
[0092] Dissolve N-hexadecyl-4-bromo-5-nitro-1,8-naphthalimide...
Embodiment 3
[0104] Synthesis of cell membrane probe MBSO3-DAC.
[0105] Synthesis of intermediate MBAN-NBr:
[0106]
[0107] 4-Bromo-5-nitro-1,8-naphthalimide (0.50g, 1.56mmol) was dissolved in 60mL of ethanol, and 1-(N-(3-amino)propyl-N- Methyl)amino-3,7-dimethyloctane (1.07g, 4.68mmol), heated to 70°C for 12h, then distilled off the solvent under reduced pressure, and the residue was passed through a silica gel column (dichloromethane / methanol=80 : 1, V / V) isolated yellow solid MBAN-NBr 0.43g, yield 52%.
[0108] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0109] 1 H NMR (400MHz, CDCl 3 )δ8.68(d, J=7.6Hz, 1H), 8.48(d, J=7.7Hz, 1H), 8.20(d, J=7.8Hz, 1H), 7.92(d, J=7.6Hz, 1H) ,4.24(t,J=6.5Hz,2H),2.80(s,2H),2.65(s,2H),2.46(s,3H),2.10(s,2H),1.41(s,2H),1.30– 1.03(m,8H),0.86(t,J=6.3Hz,9H).
[0110] Its nuclear magnetic spectrum carbon spectrum data are as follows:
[0111] 13 C NMR (101MHz, CDCl 3 )δ162.87,162.12,151.31,135.98,132.38,131.32,130.52,125....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Uv absorption wavelength | aaaaa | aaaaa |
| Molar extinction coefficient | aaaaa | aaaaa |
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com