Indole heterocyclic compound, preparation method thereof and application of indole heterocyclic compound in prevention and treatment of plant diseases
A technology of heterocyclic compounds and compounds, applied in the directions of botanical equipment and methods, applications, plant growth regulators, etc., to achieve the effect of good anti-plant virus and pathogenic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
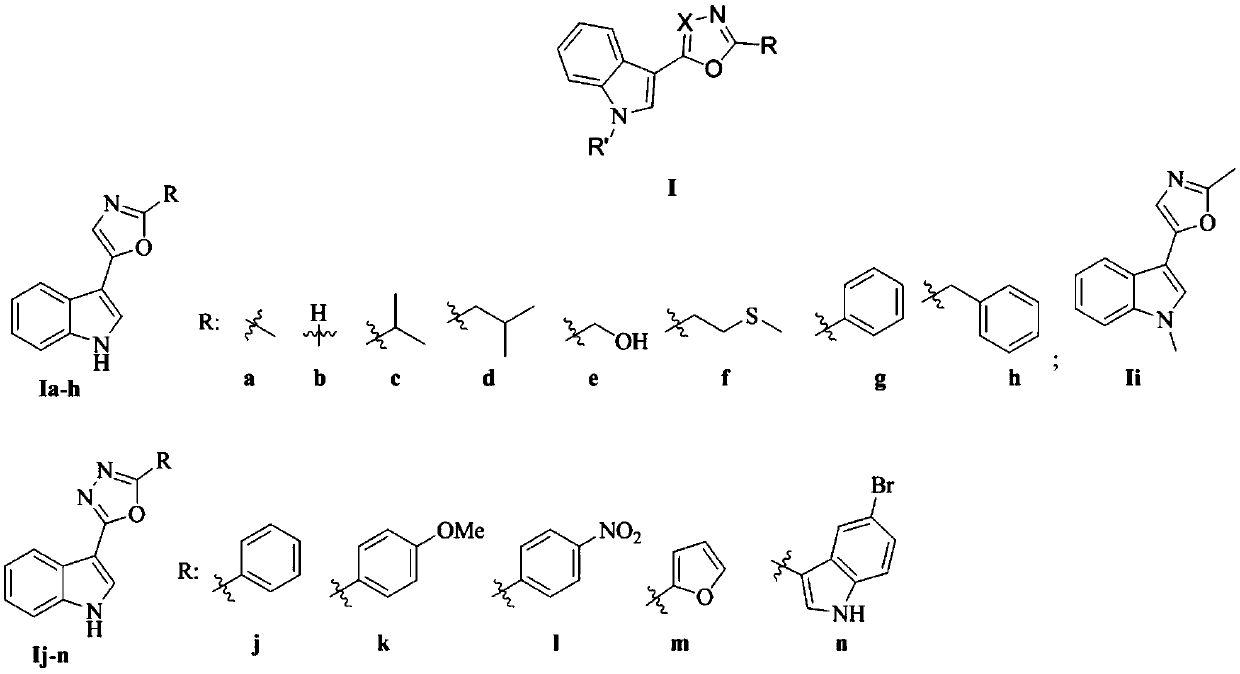
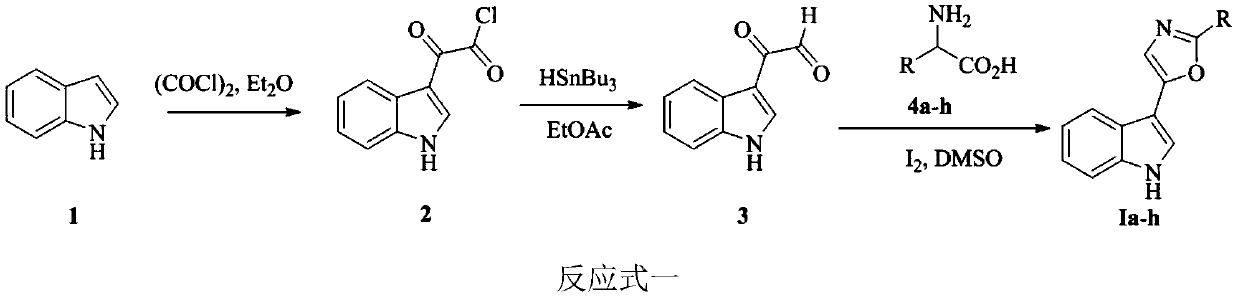
[0046] Embodiment 1: the synthesis of indole heterocyclic compound Ia-h
[0047]
[0048] Indole carbonyl acetyl chloride: Weigh 20g of indole (marked as 1) into a 1L four-necked flask, add anhydrous ether (Et 2(0) 350mL, after the solid is completely dissolved, slowly add oxalyl chloride [(COCl) dropwise at 0°C 2 】17.3mL, 30min to complete the addition. The reaction solution was reacted at 0° C. for 3 h, then raised to room temperature and then reacted for 1 h. After the reaction was completed, it was filtered with suction and washed three times with anhydrous ether. After drying, 32 g of compound 2 (ie, indolecarbonyl acetyl chloride) was obtained, with a yield of 90%, which could be directly used in the next reaction after being characterized.
[0049] Indolecarbonylacetaldehyde: Add the product 2 prepared above into a 500mL four-necked round-bottomed flask, then add 153mL of ethyl acetate, and add 42.9mL of tri-n-butyltin hydride dropwise in a nitrogen atmosphere at ...
Embodiment 2
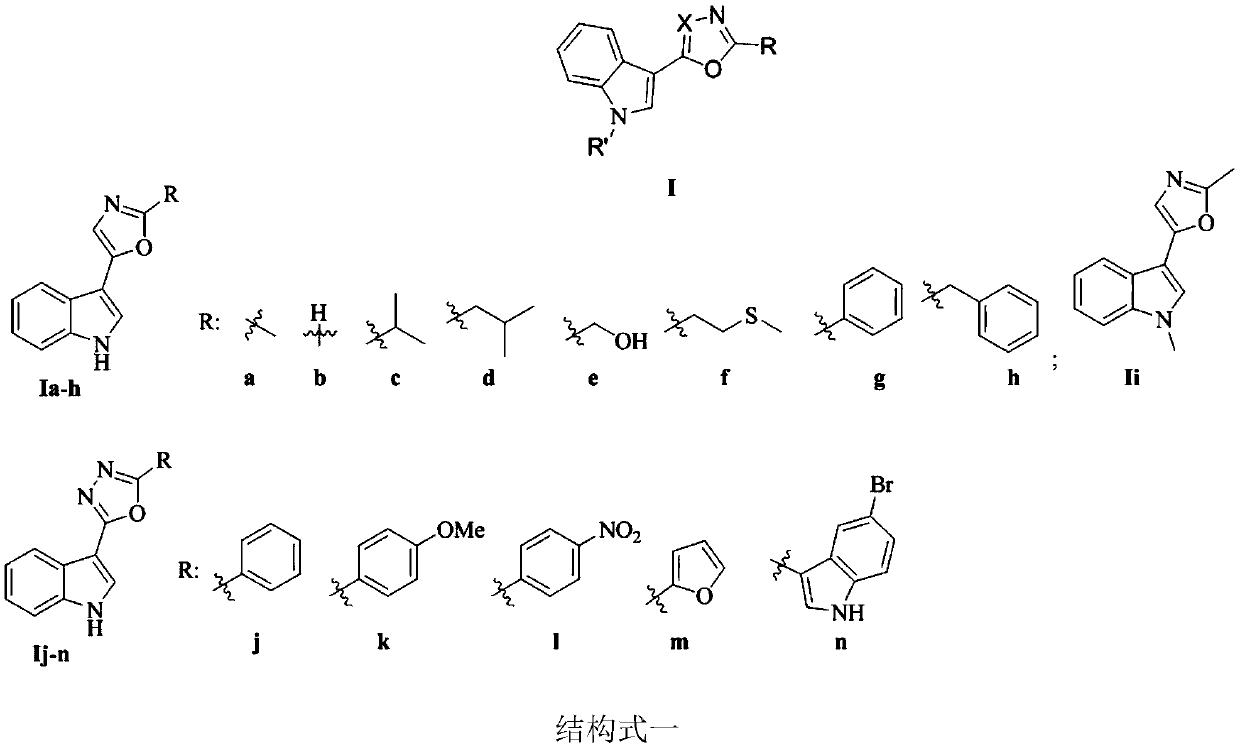
[0059] Embodiment 2: the synthesis of indole heterocyclic compound Ii
[0060]
[0061] Take compound Ia (0.4g), add 40mL of tetrahydrofuran, add 60% NaH (0.16g) under mechanical stirring under nitrogen atmosphere at room temperature, react at room temperature for half an hour, then add 0.86g methyl iodide, react at room temperature for 1h, and heat to reflux for 1h. Add 10mL of water and react for 5min. Extraction, drying and finally concentration of the resulting product Ii. Yellow solid, yield 86%, melting point 71-73°C; 1 H NMR (400MHz, DMSO-d 6)δ7.86(d, J=7.8Hz, 1H, Ar-H), 7.73(s, 1H, Ar-H), 7.50(d, J=8.1Hz, 1H, Ar-H), 7.30(s, 1H, Ar-H), 7.27(d, J=7.5Hz, 1H, Ar-H), 7.19(d, J=7.5Hz, 1H, Ar-H), 3.83(s, 3H, N-CH 3 ),2.48(s,3H,Ar-CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ158.3, 147.0, 136.8, 126.8, 123.7, 122.1, 120.1, 119.6, 119.2, 110.3, 103.0, 32.6, 13.5; HRMS (ESI) calcd for C 13 h 13 N 2 O(M+H) + 213.1022, found 213.1027.
Embodiment 3
[0062] Embodiment 3: the synthesis of indole heterocyclic compound Ij-n
[0063]
[0064] Indolehydrazone 7a-e:
[0065] Add indole-3-carboxylhydrazide (6mmol), the corresponding aldehyde (6mmol), and absolute ethanol (80mL) to a 100mL round-bottomed flask equipped with a magnetic stirrer, and react the mixture at 80°C for 1 hour, then cool Return to room temperature, use a rotary evaporator to evaporate until 15-20 mL of liquid remains, let it stand, and filter with suction to obtain acylhydrazones 7a-e, which are detected by NMR, and the data are as follows.
[0066] 7a. White solid, melting point 233-234°C, yield 79%; 1 H NMR (400MHz, DMSO-d 6 )δ11.74(s,1H,NH),11.38(s,1H,NH),8.20–8.33(m,3H,Ar-H),7.72(d,J=7.3Hz,2H,Ar-H), 7.47(s,1H,Ar-CH),7.38–7.49(m,3H,Ar-H),7.14–7.22(m,2H,Ar-H); 13 C NMR (100MHz, DMSO-d 6 )δ134.7, 129.5, 128.8, 126.7, 122.2, 120.8, 111.9.
[0067] 7b. White solid, melting point 242-243°C, yield 90%; 1 H NMR (400MHz, DMSO-d 6 )δ11.72(s,1H,NH),11.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com