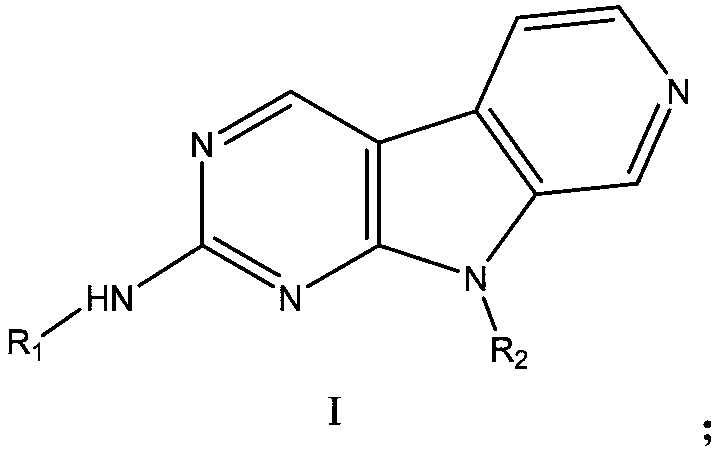
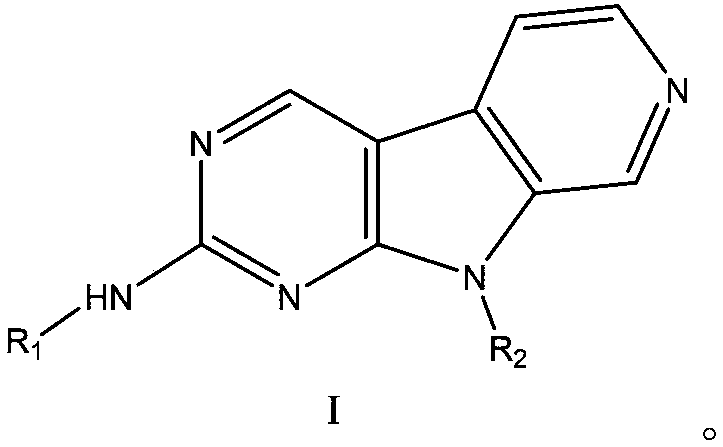
CDK4-FLT3 inhibitor and application thereof
A solvate and compound technology, applied in the field of CDK4-FLT3 inhibitors, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
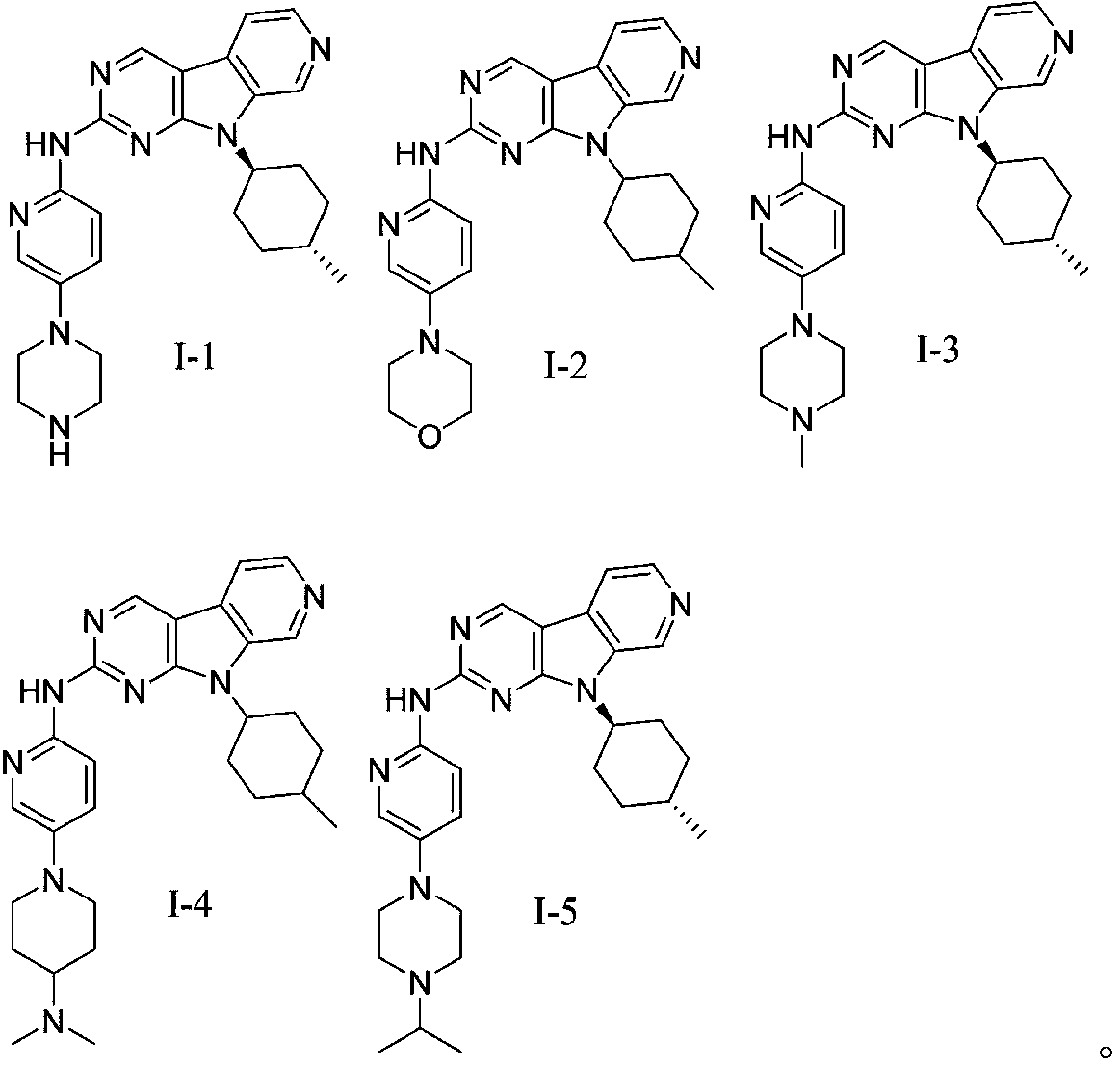
[0067] The preparation of embodiment 1 compound I-1
[0068]
[0069] Compound I-1A (281 mg, 1 mmol), compound I-1B (197 mg, 1 mmol) were dissolved in 1,4-dioxane (60 mL), and tris(dibenzylideneacetone) dipalladium (92 mg, 0.1 mmol), 2-dicyclohexylphosphorus-2',4',6'-triisopropylbiphenyl (95mg, 0.2mmol) and cesium carbonate (2.09g, 6.4mmol), heated to 95°C~ The reaction was stirred at 100°C for 15 hours. Cool to room temperature, concentrate, add water to quench, separate layers, extract the aqueous phase with ethyl acetate (100mL×2), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate, and the crude product is subjected to silica gel column chromatography Purification (methanol:dichloromethane=1 / 20 (V / V), add appropriate amount of 0.2% triethylamine) to obtain the compound represented by formula I-1 (293mg), yield 66.2%, HPLC purity 98.5%.
[0070] m / z:443(M+H) + .
Embodiment 2
[0071] The preparation of embodiment 2 compound 1-2
[0072]
[0073] The preparation method of compound I-2 was tested in a manner similar to that of Example 1, compound I-2A (281 mg, 1 mmol) and compound I-2B (198 mg, 1 mmol). The crude product was purified by silica gel column chromatography (methanol: dichloromethane = 1 / 20 (V / V), plus an appropriate amount of 0.2% triethylamine) to obtain the compound shown in formula I-2 (243mg), yield 54.9%, HPLC 99.2% purity.
[0074] m / z:444(M+H) + .
Embodiment 3
[0075] The preparation of embodiment 3 compound 1-3
[0076]
[0077] The preparation method of compound 1-3 was experimented in a manner similar to that of Example 1. The crude product was purified by silica gel column chromatography (methanol: dichloromethane = 1 / 20 (V / V), plus an appropriate amount of 0.2% triethylamine) to obtain the compound shown in formula I-3 with a yield of 56.0% and a HPLC purity of 96.8% .
[0078] m / z:457(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com