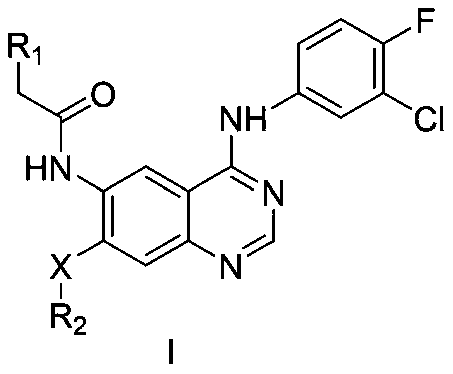
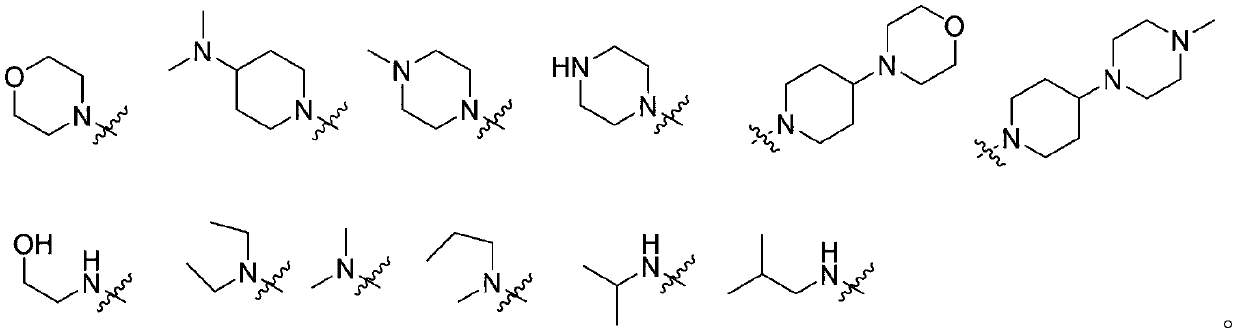
Quinazoline derivative serving as EGFR inhibitor and application of derivative
A drug and compound technology, applied in the field of biomedicine, can solve the problems of drug resistance and poor selectivity of EGFR inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
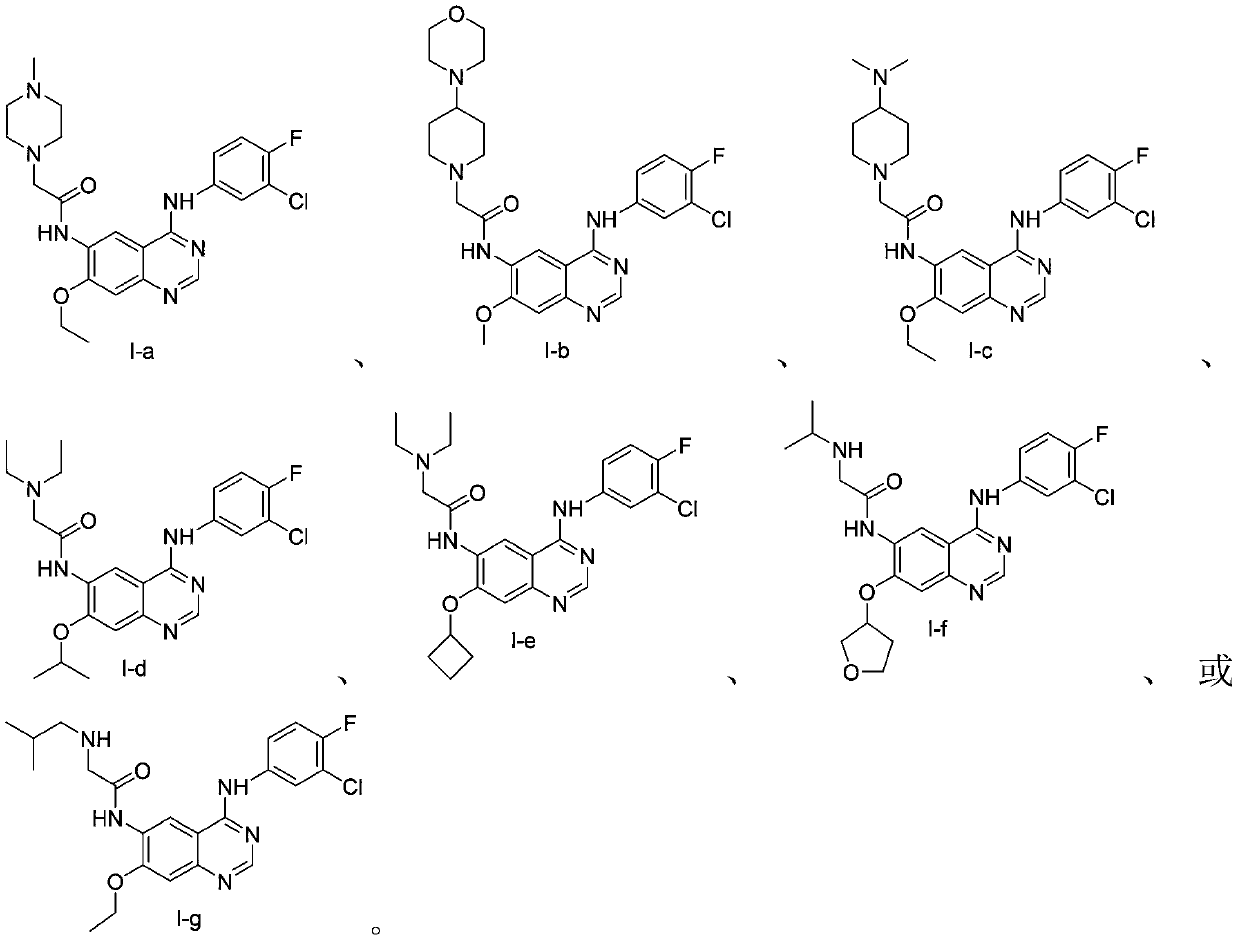
[0080] The preparation of embodiment 1 compound I-a
[0081]
[0082] first step:
[0083] Compound shown in formula I-a-1 (1.46g, 5.77mmol) was dissolved in 60mL isopropanol, compound (840mg, 5.77mmol) and p-toluenesulfonic acid (993mg, 5.77mmol) shown in formula 2 were added thereto, and The mixture was stirred and reacted at 75°C for 5 hours. After the reaction was detected by TLC, the solvent was evaporated under reduced pressure, and the residual solid was dispersed in 200 mL of ethyl acetate, washed with saturated aqueous sodium bicarbonate, water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure A crude product was obtained, which was separated by column chromatography to obtain the compound shown in formula I-a-3 (yield 1.59 g, yield 76%).
[0084] Step two:
[0085] The compound shown in formula I-a-3 (290 mg, 0.8 mmol) was placed in a 100 mL hydrogenation bottle, 50 mL of methanol was added, and after nitrogen rep...
Embodiment 2
[0092] The preparation of embodiment 2 compound I-b
[0093]
[0094] The preparation method of compound I-b was carried out in a manner similar to that of Example 1, except that the starting materials were changed to compound I-b-1 and compound I-b-7. Purification by silica gel chromatography gave the compound represented by formula I-b (yield 66%, HPLC purity 99.6%).
[0095] m / z:530(M+H) + .
Embodiment 3
[0096] The preparation of embodiment 3 compound I-c
[0097]
[0098] The preparation method of compound I-c was experimented in a manner similar to that of Example 1, except that the starting material was changed to compound I-c-7. Purification by silica gel chromatography gave the compound represented by formula I-c (yield 52%, HPLC purity 99.2%).
[0099] m / z:502(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com