Method for transferring C=N double bonds by chlorella
A technology of chlorella and Schiff base, applied in the field of chemistry, can solve the problems of environmental pollution and high cost, and achieve the effects of environmental friendliness, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
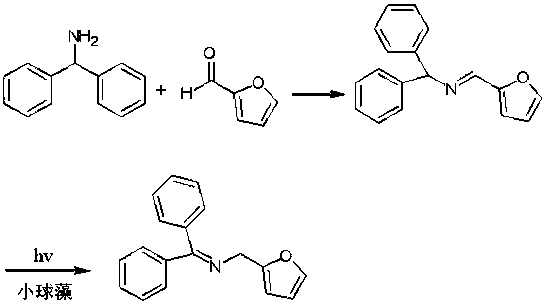
[0020] In the first step, furfural (0.961 g, 10.0 mmol), diphenylmethylamine (1.22 g, 6.7 mmol), and dichloromethane (9.61 g) were added to the reaction flask, and the reaction was refluxed, and the end point of the reaction was tracked by TLC. When the diphenylmethylamine is consumed, stop heating, distill under reduced pressure, and recrystallize the concentrate from ethanol to obtain the Schiff base formed by furfural and diphenylmethylamine.
[0021] In the second step, the Schiff base (1.00 g, 5.5 mmol), chlorella (5 g), and ethyl acetate (20.00 g) formed by the furfural obtained in the first step and diphenylmethylamine were added to the reaction flask, Under light at room temperature, TLC followed the reaction end point. When the spots of the Schiff base raw material formed by furfural and diphenylmethylamine completely disappear, the C=N double bond of the Schiff base is completely transferred, and the light is stopped to obtain the product after the transfer of the C=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com