Preparation method of hydroxymethyl-substituted aromatic heterocyclic compound
A technology of aromatic heterocycle and hydroxymethyl group, which is applied in the fields of optoelectronics and organic semiconductor materials science, medicinal chemistry, and synthetic chemistry, can solve the problems of explosive hazardous chemicals and other problems, and achieve simple methods and avoid explosive hazardous chemicals and the effects of the use of precursor chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
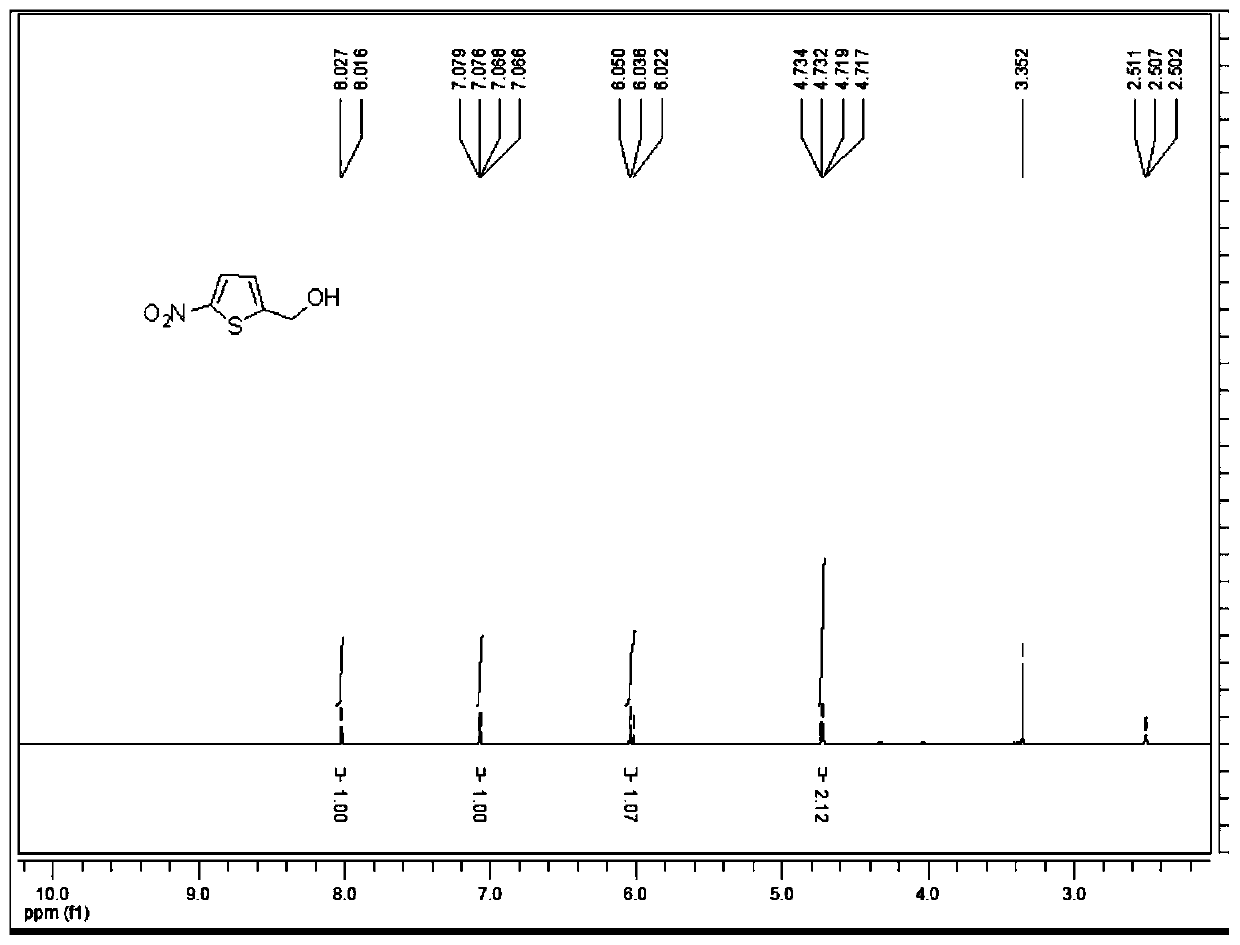
[0035] This embodiment provides the synthesis of 2-hydroxymethyl-5-nitrothiophene, the method is as follows:
[0036] Synthesis using borane dimethyl sulfide in tetrahydrofuran:
[0037] Under nitrogen protection, compound 5-nitrothiophene-2-carboxylic acid (0.86g, 4.97mmol) was added to dry tetrahydrofuran (10mL), and 1.0M borane tetrahydrofuran solution (14.8mL, 14.8mmol) was slowly added dropwise under ice-cooling , after the dropwise addition was completed, the temperature was raised to room temperature under the protection of nitrogen, and stirred for 16 hours. Add methanol 10mL under ice bath to quench, and extract with ethyl acetate (a total of 3 extractions, using 40mL ethyl acetate each time), combine the organic phases, wash with saturated sodium bicarbonate solution, water, saturated brine successively, anhydrous sulfuric acid Sodium-dried, filtered, and the filtrate was evaporated to dryness and purified by a rotary evaporator to obtain a brown oily substance, whi...
Embodiment 2
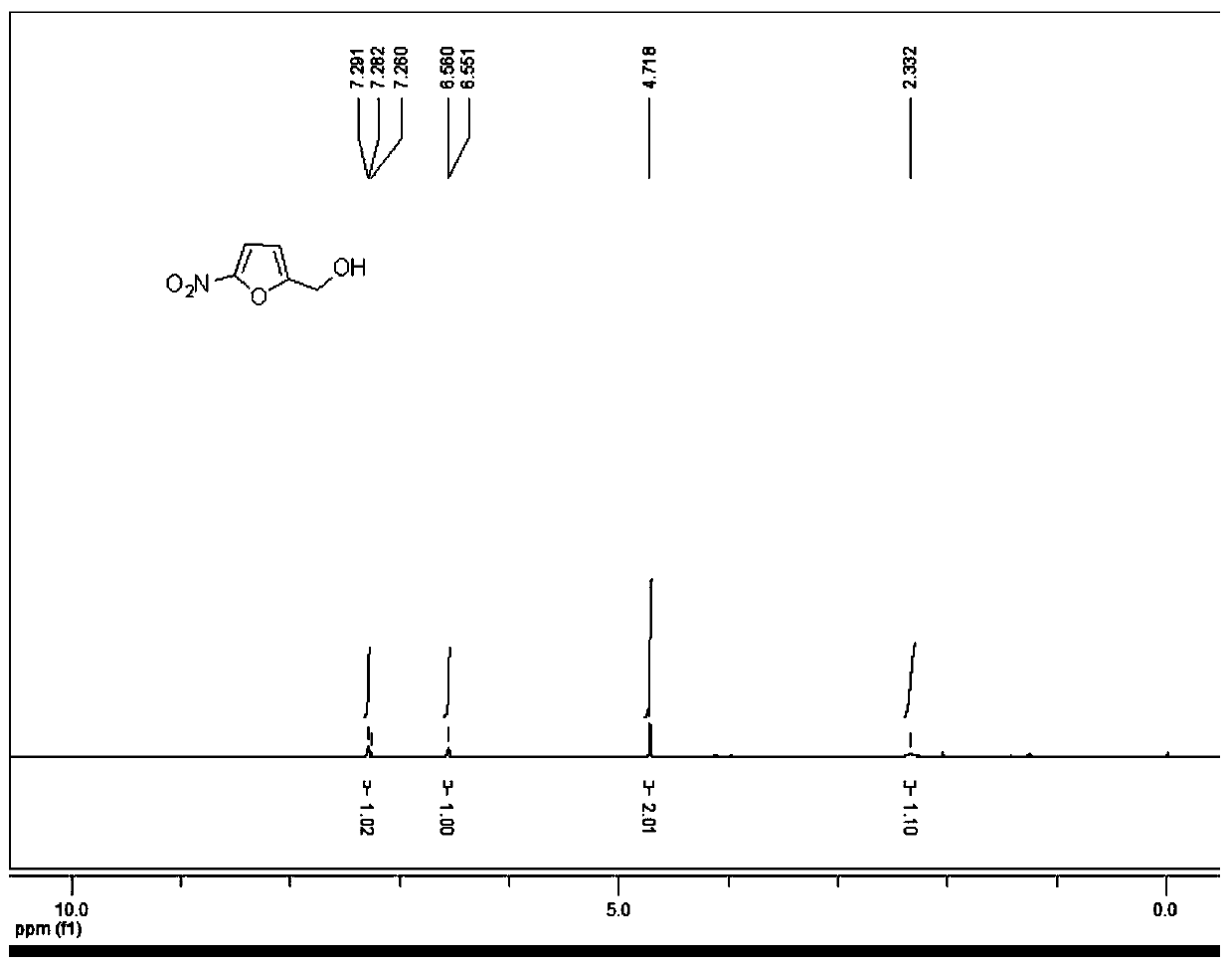
[0043] This embodiment provides the synthesis of 2-hydroxymethyl-5-nitrofuran, the method is as follows:
[0044] Under nitrogen protection, the compound 5-nitrofuran-2-carboxylic acid (1.00g, 6.37mmol) was added to dry tetrahydrofuran (12mL), and 1.0M borane tetrahydrofuran solution (19.2mL, 19.2mmol) was slowly added dropwise under ice-cooling , after the dropwise addition was completed, the temperature was raised to room temperature under the protection of nitrogen, and stirred for 16 hours. Add methanol 10mL under ice bath to quench, extract with ethyl acetate (40mL×3), combine the organic phases, wash with saturated sodium bicarbonate solution, water, saturated brine successively, dry over anhydrous sodium sulfate, filter, and the filtrate is evaporated by rotary evaporation After evaporation to dryness and purification, a brown oily substance, 2-hydroxymethyl-5-nitrofuran (0.89 g, yield 98%) was obtained.
[0045] m / z: 160.0[M+H]+HNMR: 1H-NMR (Bruker 400MHz, CDCl 3 ) δ...
Embodiment 3
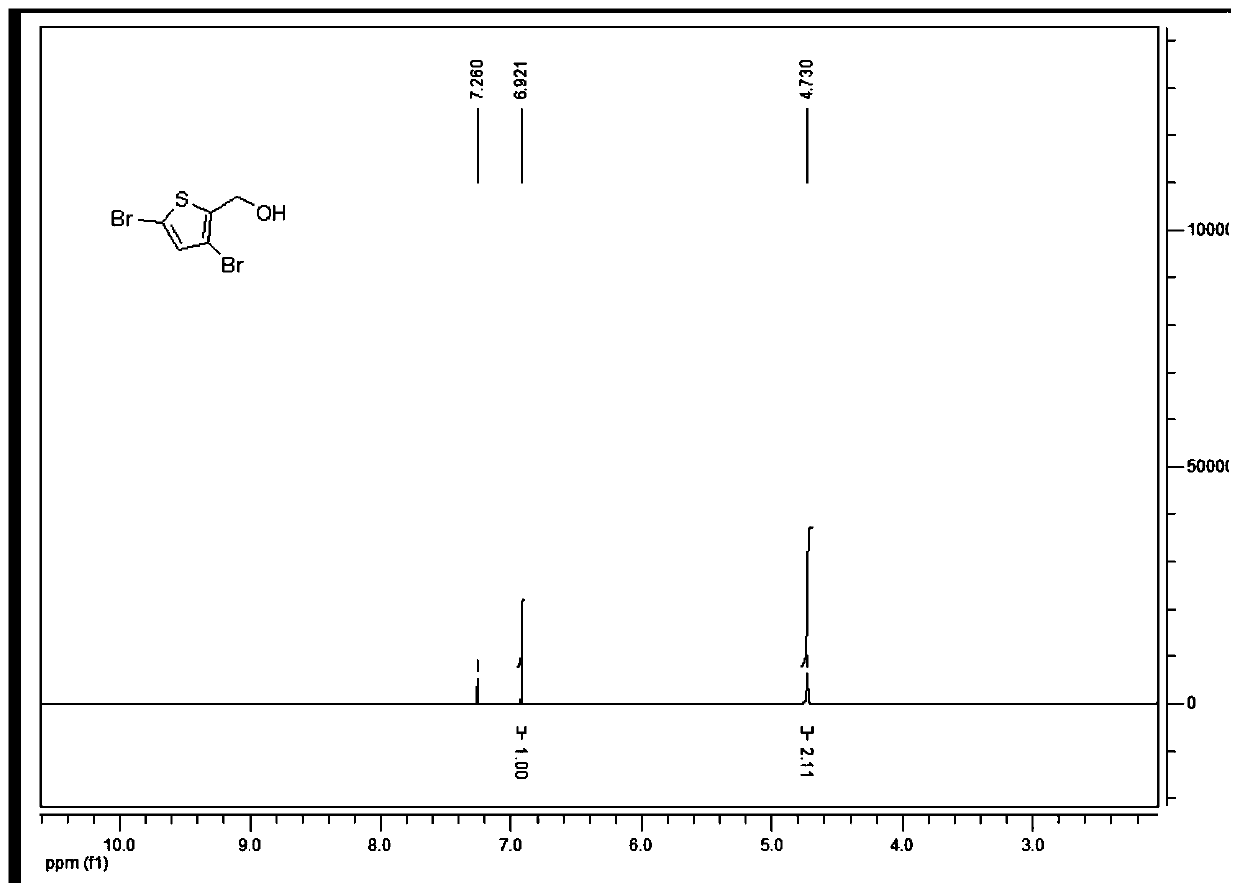
[0050] This example provides the synthesis of 2-hydroxymethyl-3,5-dibromothiophene, which is synthesized using a THF solution of borane dimethyl sulfide, and the method is as follows:
[0051] Under nitrogen protection, compound 3,5-dibromothiophene-2-carboxylic acid (1.00g, 3.5mmol) was added into dry tetrahydrofuran (10mL), and 1.0M borane tetrahydrofuran solution (10.5mL, 10.5 mmol), after the dropwise addition was completed, the mixture was warmed up to room temperature and stirred for 16 hours under nitrogen protection. Add methanol 10mL under ice bath to quench, and extract with ethyl acetate (a total of 3 extractions, using 40mL ethyl acetate each time), combine the organic phases, wash with saturated sodium bicarbonate solution, water, saturated brine successively, anhydrous sulfuric acid Sodium-dried, filtered, and the filtrate was evaporated to dryness and purified by a rotary evaporator to obtain a light yellow oil, namely 2-(hydroxymethyl)-3,5-dibromothiophene (0.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com