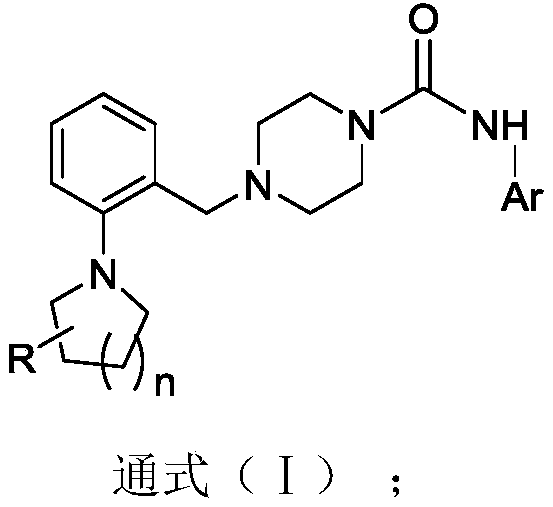
4-(2-(pyrrolidine/piperidine-1-yl)benzyl)-piperazine urea TRPV1 antagonist and preparation method and application thereof
A technology of pyrrolidine and pyrrolidine, which is used in the preparation of analgesic drugs to treat pain, in the field of 4-benzyl)-piperazine urea novel TRPV1 antagonists, which can solve problems such as elevated body temperature and achieve strong effects , No side effects of elevated body temperature, analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of N-(4-methyl-2-nitrophenyl)-4-(2-(pyrrolidinyl-1-yl)benzyl)piperazine-1-carboxamide (1)
[0060]
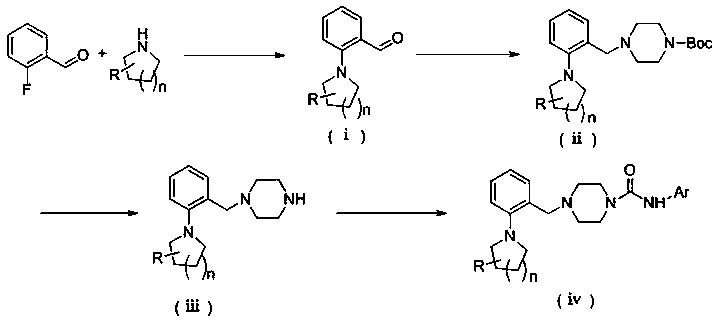
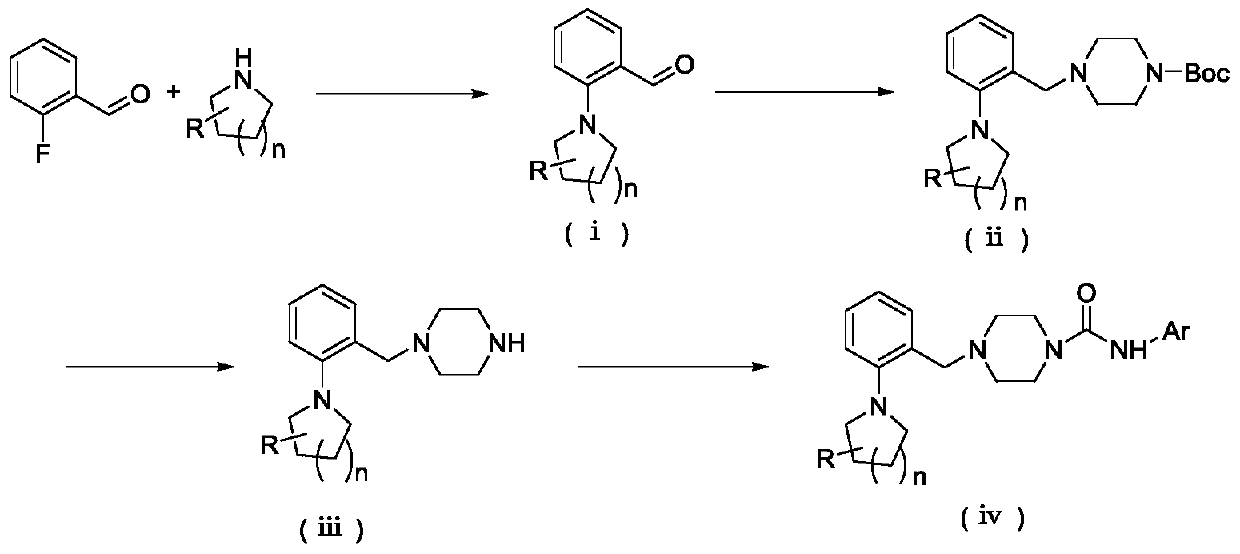
[0061] (a) Preparation of 2-(pyrrolidin-1-yl) benzaldehyde
[0062] Accurately weigh anhydrous potassium carbonate (2.7840g, 20.143mmol), put it into a 100mL eggplant-shaped bottle, stir and dissolve 10mL dimethyl sulfoxide, add 2-fluorobenzaldehyde (1.0g, 8.057mmol) and tetrahydro Pyrrole (0.859 g, 12.085 mmol). Reacted overnight in an oil bath at 85°C. After the reaction was detected by TLC, extracted with ethyl acetate (30mL×2), combined the organic phases and washed with water (30mL×3), evaporated the solvent under reduced pressure to obtain 2-(pyrrolidine-1 -yl) benzaldehyde.
[0063] (b) Preparation of 4-(2-(pyrrolidin-1-yl) benzyl) piperazine-1 carboxylic acid tert-butyl ester
[0064] At room temperature, add 2-(pyrrolidin-1-yl)benzaldehyde (1.3697g, 7.8165mmol) and 20mL dichloromethane into a 100mL one-necked flask and stir to dissol...
Embodiment 2
[0070] Example 2: Preparation of N-(4-chloro-2-nitrophenyl)-4-(2-(pyrrolidin-1-yl)benzyl)piperazine-1-carboxamide (2)
[0071]
[0072] During the preparation process, the 4-methyl-2-nitroaniline in Example 1 was replaced with 4-chloro-2-nitroaniline, and other references were made to the preparation method in Example 1 to obtain compound 2 and obtain a red solid. Yield 13.9%. The experimental data are as follows:
[0073] C 22 h 26 ClN 5 o 3 ; yield: 13.9%; red solid; m.p=54.8-55.8°C; 1 H NMR (CDCl 3 ,300MHz): δppm10.15(s,1H,NH),8.66(d,1H,J=9.0Hz,Ar-H),8.19(d,1H,J=2.7Hz,Ar-H),7.55(dd ,1H,J=3.0,9.0Hz,Ar-H),7.41(dd,1H,J=3.0,9.0Hz,Ar-H),7.19(t,1H,J=9.0Hz,Ar-H),6.96 -6.91(m,2H,Ar-H),3.59(t,5H,J=4.5Hz,Ar-CH 2 ,pyrrolidine), 3.19(t,3H,J=3.0Hz,piperazine),2.54(t,3H,J=6.0Hz,piperazine),1.94-1.90(m,3H,pyrrolidine),1.49-1.33(m,2H ,pyrrolidine),1.28-1.22(m,2H,pyrrolidine); HRMS m / z:[M+H] + 444.1805 (calcd 444.1797).
Embodiment 3
[0074] Example 3: Preparation of 4-(2-(pyrrolidinyl-1-yl)benzyl)-N-(2-(trifluoromethyl)phenyl)piperazine-1-carboxamide (3)
[0075]
[0076] During the preparation process, the 4-methyl-2-nitroaniline in Example 1 was replaced with 2-trifluoromethylaniline, and other references were made to the preparation method in Example 1 to obtain compound 3 to obtain a reddish-brown solid, producing rate of 50.4%. The experimental data are as follows:
[0077] C 23 h 27 f3 N 4 O; yield: 50.4%; refous solid; m.p=102.2-103.2°C; 1 H NMR (CDCl 3 ,300MHz): δppm8.11(d,1H,J=9.0Hz,Ar-H),7.53(q,2H,J=9.0Hz,Ar-H),7.42(dd,1H,J=9.0,3.0Hz ,Ar-H),7.21-7.10(m,2H,Ar-H),6.91(q,2H,J=9.0Hz,Ar-H),6.80(s,1H,NH),3.58(s,2H, Ar-CH2), 3.51(t, 4H, J=4.5Hz, pyrrolidine), 3.20(t, 4H, J=6.0Hz, piperazine), 2.52(t, 4H, J=4.5Hz, piperazine), 1.94-1.88 (m,4H,pyrrolidine); HRMSm / z:[M+H] + 433.2207 (calcd. 433.2210).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com