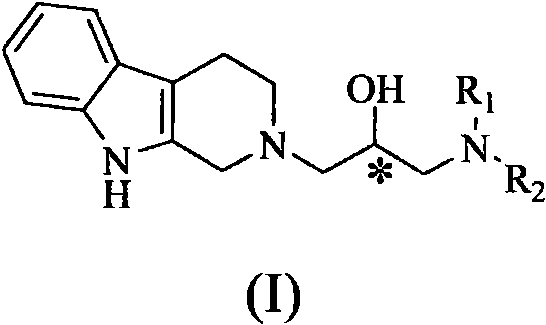
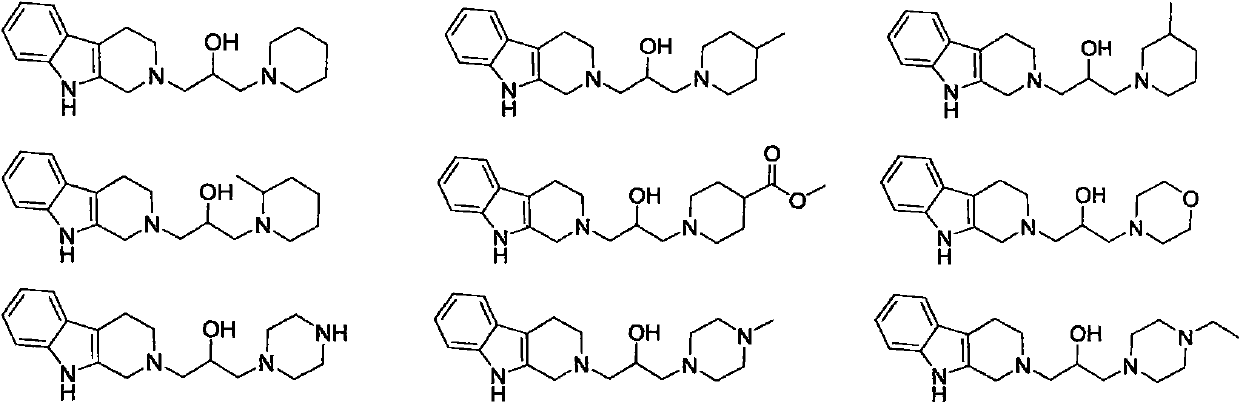
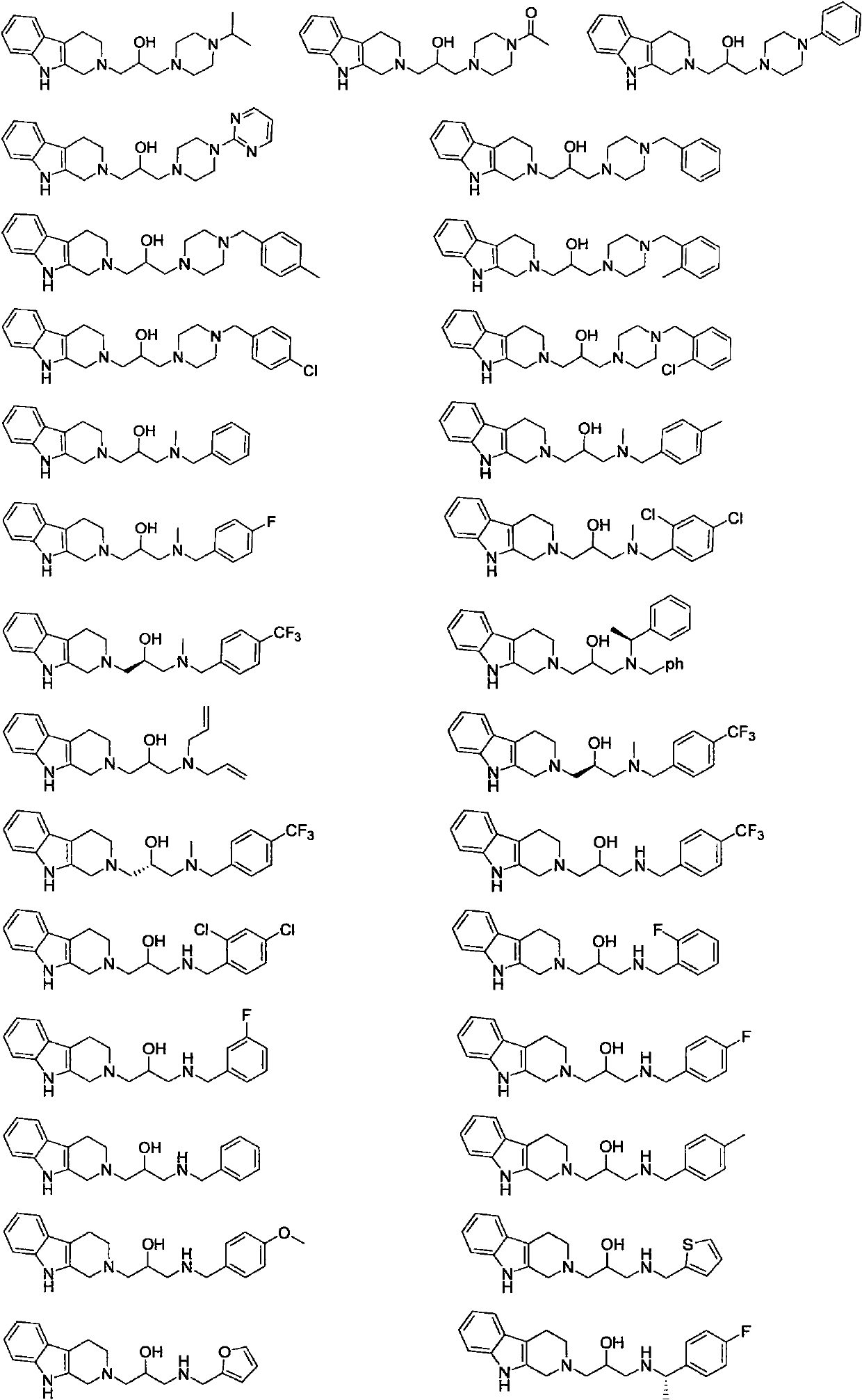
Isopropanolamine substructure-containing 1, 2, 3, 4-tetrahydro-beta-carboline compound and preparation method and application thereof
A technology of propanolamine sub-compounds, which is applied in the field of medicinal chemistry and can solve problems such as instability to light, complex structure and difficulty in synthesis, and the use of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of intermediate 2-(oxyethylene-2-methylene)-1,2,3,4-tetrahydro-β-carboline
[0046] 1,2,3,4-tetrahydro-β-carboline (5.0g, 28.45mmol), K 2 CO 3 (0.95g, 34.14mmol) and 30mL DMF were added to a 100mL round-bottomed flask, stirred in an ice bath for 10min, then epibromopropane (1.05g, 37.05mmol) was slowly added, and reacted at room temperature for 24h to end the reaction. The reaction was quenched with water, extracted with ethyl acetate, washed with saturated ammonium chloride solution, the organic phase was taken, dried over anhydrous sodium sulfate, precipitated, and column chromatographed to obtain a white solid with a yield of 37.9%. Its NMR data are: 1 H NMR (500MHz, CDCl 3 )δ7.98(s, 1H, -NH), 7.46(d, J=7.7Hz, 1H, Ar-H), 7.26(d, J=7.9Hz, 1H, Ar-H), 7.12(dt, J =7.1, 1.3Hz, 1H, Ar-H), 7.07(dt, J=7.8, 1.1Hz, 1H, Ar-H), 3.88-3.82(m, 1H, -N-CH 2 -), 3.66 (dt, J=14.7, 1.5Hz, 1H, -N-CH 2 -), 3.24-3.17(m, 1H, -O-CH-), 3.05(dd, J=13.4, 2.9Hz, 1...
Embodiment 2
[0048] Example 2: 1-(piperidin-1-yl)-3-(1,2,3,4-tetrahydro-β-carbolin-2-yl)-propan-2-ol
[0049] Put 2-(oxyethylene-2-methylene)-1,2,3,4-tetrahydro-β-carboline (0.23g, 1.0mmol), benzylamine (2.0mmol) and 5mL absolute ethanol into 15mL reaction bottle, then reacted at 60°C, followed by TLC until 2-(oxyethylene-2-methylene)-1,2,3,4-tetrahydro-β-carboline was completely consumed, and the reaction was terminated. The reaction was quenched by adding 20 mL of water, and extracted twice with 30 mL of dichloromethane, the organic phase was collected, dried over anhydrous sodium sulfate, precipitated, and column chromatographed to obtain a white solid with a yield of 91.9%.
Embodiment 3
[0050] Example 3: 1-(benzylamino)-3-(1,2,3,4-tetrahydro-β-carbolin-2-yl)propan-2-ol converts 2-(oxyethylene-2-methylene Base)-1,2,3,4-tetrahydro-β-carboline (0.23g, 1.0mmol), potassium carbonate (0.3mmol), piperidine (1.1mmol) and 6mL isopropanol were put into 15mL in one pot Then react at room temperature, TLC tracking, until 2-(oxyethylene-2-methylene)-1,2,3,4-tetrahydro-β-carboline is completely consumed, the reaction is terminated. The reaction was quenched by adding 20 mL of water, and extracted twice with 30 mL of dichloromethane, the organic phase was collected, dried over anhydrous sodium sulfate, precipitated, and column chromatographed to obtain a white solid with a yield of 95.7%.
[0051] The following compounds were prepared by the steps similar to the above examples, only replacing the corresponding raw materials. The structure and H-NMR and C-NMR data of the compound are shown in Table 1, and their physicochemical properties are shown in Table 2.
[0052] H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com