Preparation method of mivacurium chloride and injection thereof
A technology of mivacurium chloride and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as stability and safety issues that have not been properly resolved , to achieve the effect of good long-term storage stability, less impurities, and less impurity components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
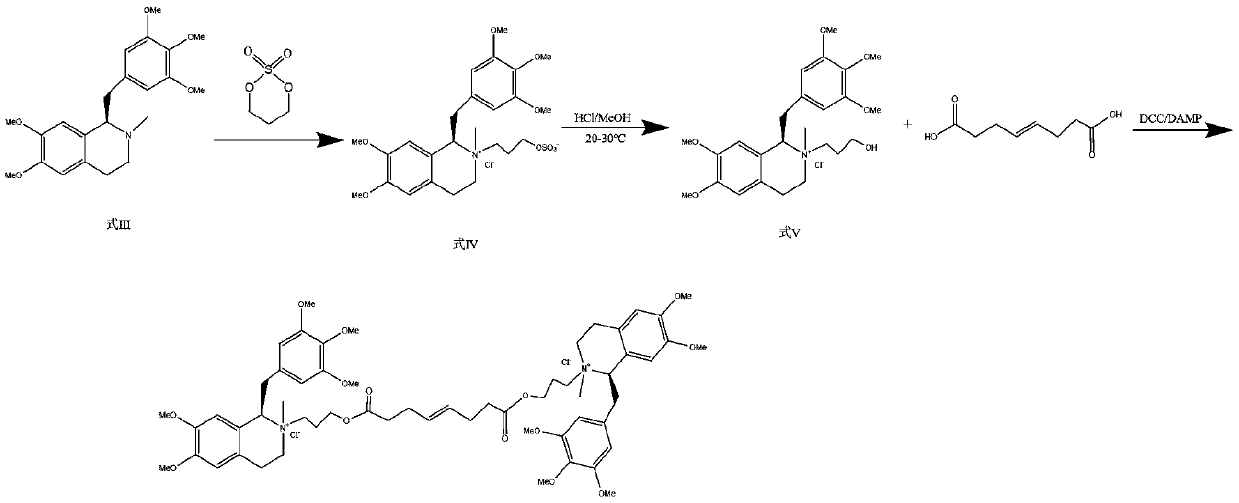
[0052] A method for synthesizing micuronium chloride, including the following steps:
[0053]
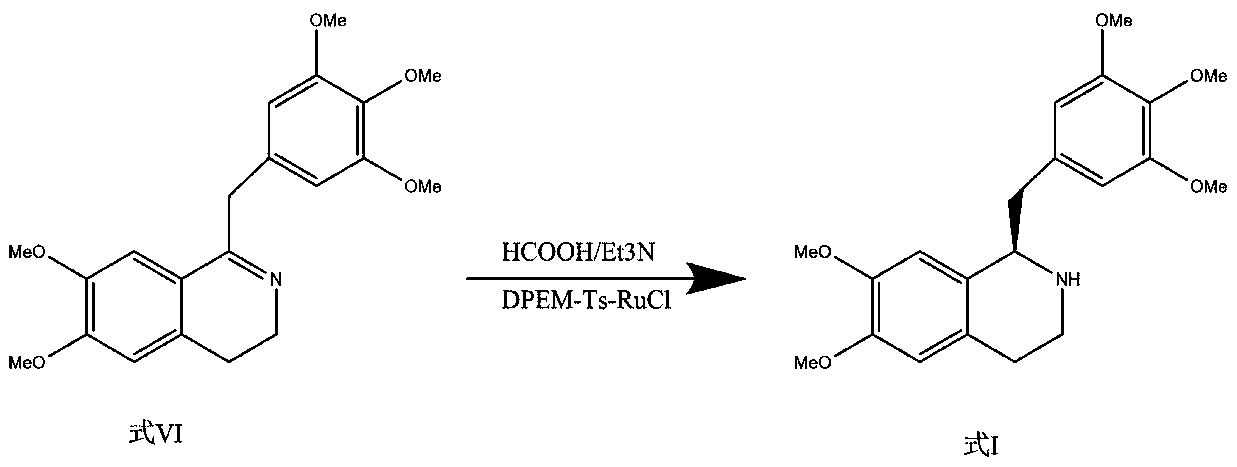
[0054] Step 1: Asymmetric catalytic reduction
[0055] The crude product (100g) of the compound of formula VI was dissolved in tetrahydrofuran (1000ml), degassed ultrasonically and replaced with nitrogen for 5 minutes, added with the metal chiral catalyst RuCl-Ts-DPEN (2500mg), formic acid-triethylamine (V / V= 5:3, 15mL), react at 15°C for 2.5 hours. Add saturated NaHCO 3 The aqueous solution (3000 mL) was quenched, and extracted with ethyl acetate (2000 mL) for three consecutive times, and the extracts were combined; the extract was washed twice with saturated brine (500 mL) and dried to obtain the compound of formula I of type R (91.9 g).
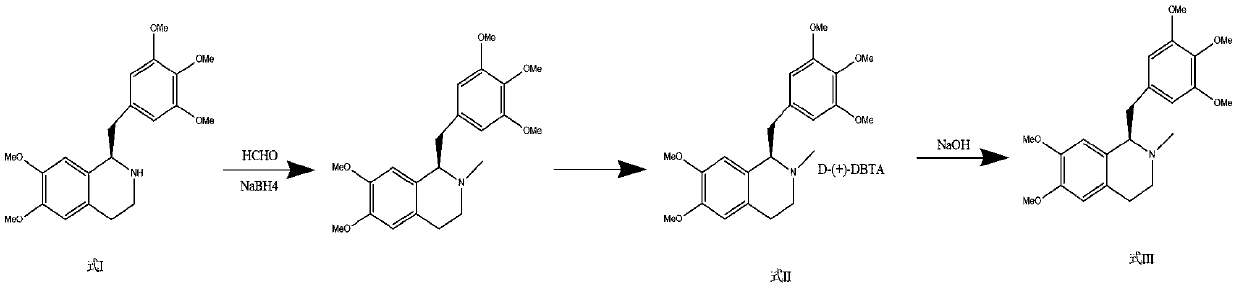
[0056] Step 2: Methylation and purification of amine
[0057] In 1500 ml of ethyl acetate, the compound of formula I (100 g), sodium borohydride (120 g), and 1200 ml of 37% formaldehyde solution were added, and refluxed until the reaction was complete....
Embodiment 2
[0063] A method for synthesizing micuronium chloride, including the following steps:
[0064] Step 1: Asymmetric catalytic reduction
[0065] The crude product (100g) of the compound of formula VI was dissolved in dichloromethane (1000ml), degassed ultrasonically, replaced with nitrogen for 5 minutes, and added with the metal chiral catalyst RuCl-Ts-DPEN (2200mg), formic acid-triethylamine (V / V=9:4, 20mL), reacted at 0°C for 7.5 hours. Add saturated NaHCO 3 The aqueous solution (3000 mL) was quenched, and extracted with dichloromethane (2000 mL) for 3 consecutive times, and the extracts were combined; the extract was washed twice with saturated brine (500 mL) and dried to obtain the R-type compound of formula I (90.3 g).
[0066] Step 2: Methylation and purification of amine
[0067] Add formula I compound (100g) and sodium borohydride (95g) to 1500ml of tetrahydrofuran, then add 1400ml of 30% formaldehyde solution, and reflux until the reaction is complete. After cooling to room t...
Embodiment 3
[0073] A method for synthesizing micuronium chloride, including the following steps:
[0074] Step 1: Asymmetric catalytic reduction
[0075] The crude product (100g) of the compound of formula VI was dissolved in methyl tert-butyl ether (1000ml), degassed ultrasonically and replaced with argon for 5 minutes, added with the metal chiral catalyst RuCl-Ts-DPEN (2000mg), formic acid-triethyl Amine (V / V=3:2, 18mL), reacted at 4°C for 6.5 hours. Add saturated NaHCO 3 The aqueous solution (2500mL) was quenched, and the extracts were continuously extracted with methyl tert-butyl ether (2000ml) for 3 times, and the extracts were combined; the extracts were washed twice with saturated brine (500ml) and dried to obtain the R-type compound of formula I (91.1g) ).
[0076] Step 2: Methylation and purification of amine
[0077] Add the compound of formula I (100g) and sodium borohydride (90g) to 1500ml of dichloromethane, and then add 800ml of 40% formaldehyde solution, and reflux until the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com