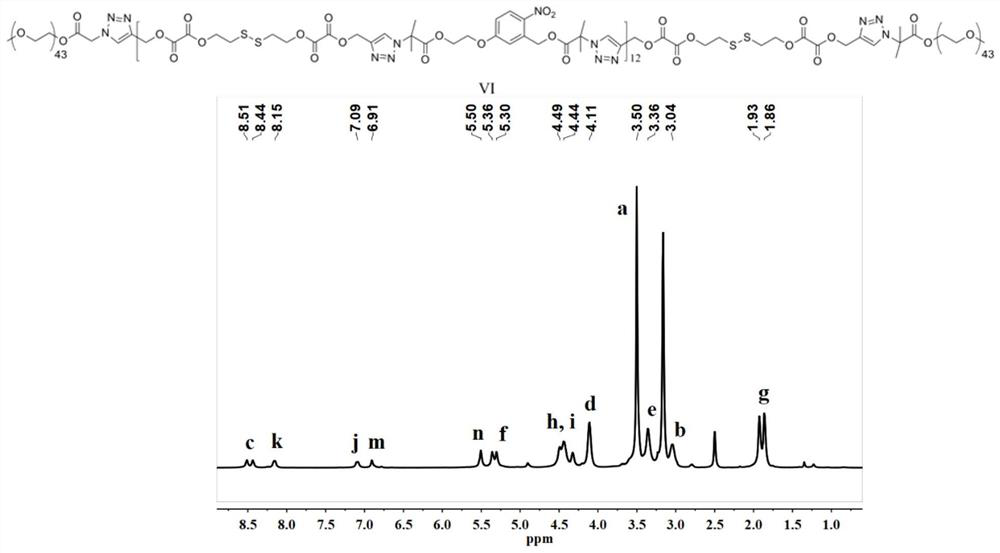
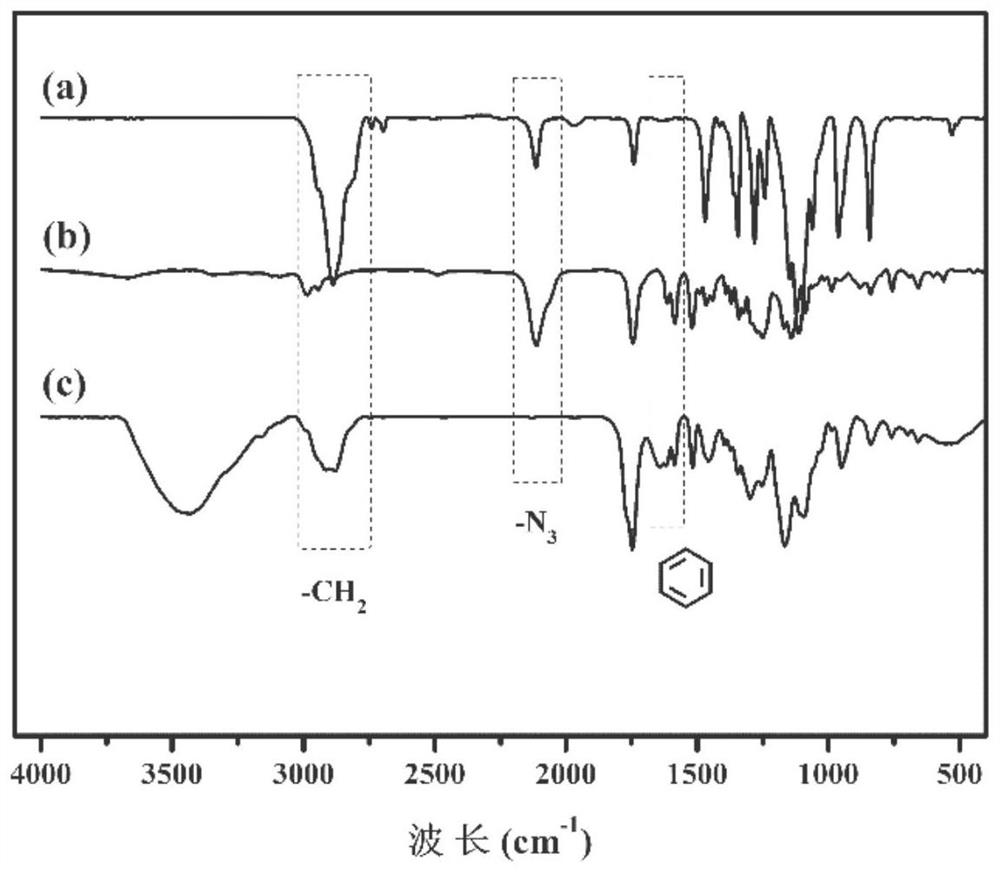
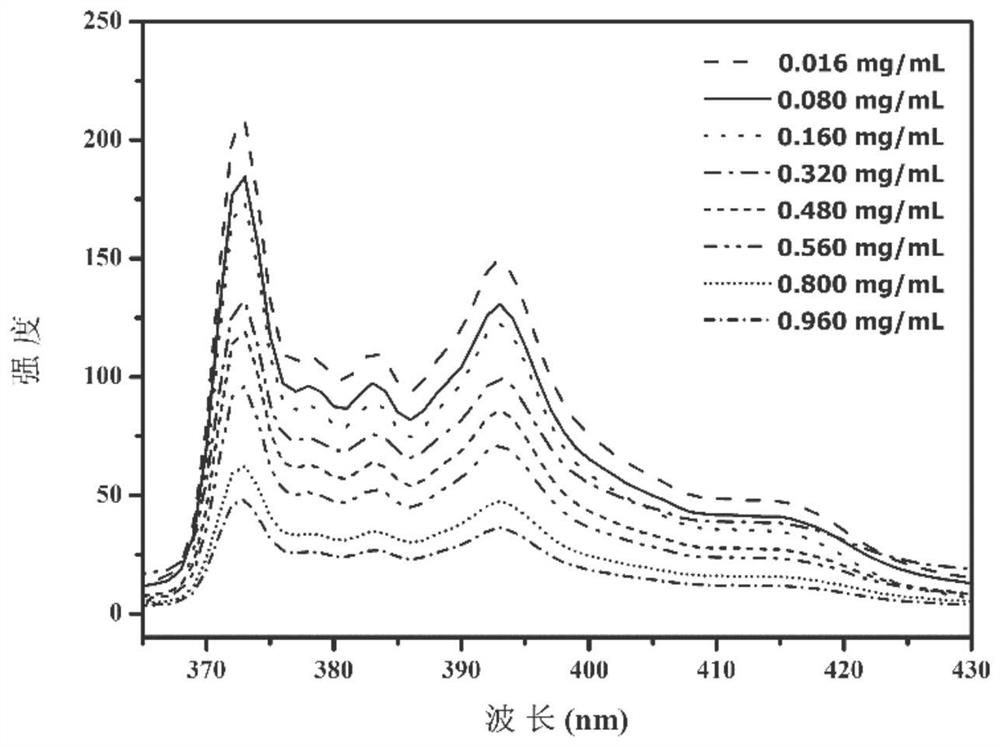
Triple stimuli-responsive block polymer micelle and its preparation method and application
A block polymer, stimuli-responsive technology, used in drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as poor therapeutic effect and inability to fully release loaded drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]
[0053] 1 Oxalyl chloride (20.00mL, 233.07mmol) and anhydrous dichloromethane (DCM, 60.00mL) was added to a 100mL one-neck round flask, 2-propyn-1-ol was added dropwise under ice-cooling (5.00 g, 89.28mmol), was added dropwise at room temperature the reaction after 3h. After reaction, the reaction solution was distilled off under reduced pressure at 98 ℃, to give a colorless transparent oily propynyloxy oxalyl chloride (8.65g, 65% yield).
[0054] Bis (2-hydroxyethyl) disulfide (1.84g, 11.93mmol), TEA (2.40g, 24.00mmol) and purified THF (10.00mL) was added to a 100mL one-neck round flask dropwise under ice propynyloxy oxalyl chloride was added (3.00g, 20.47mmol), The reaction was added dropwise at room temperature after 12h, to give a colorless transparent liquid, i.e., a compound of formula I (6.17g, 80% yield).
[0055] 2, under nitrogen, 5- (2-hydroxyethoxy) -2-nitrobenzyl alcohol (2.50g, 11.68mmol), TEA (3.05g, 29.90mmol) and THF (80.00mL) was added to 250mL three-ne...
Embodiment 2
[0064]
[0065] 1 Oxalyl chloride (22.00mL, 256.38mmol) and anhydrous dichloromethane (DCM, 60.00mL) was added to a 100mL one-neck round flask, 2-propyn-1-ol was added dropwise under ice-cooling (5.00 g, 89.28mmol), was added dropwise at room temperature after the reaction 2h. After reaction, the reaction solution was distilled off under reduced pressure at 98 ℃, to give a colorless transparent oily propynyloxy oxalyl chloride (8.72g, 66% yield).
[0066] Bis (2-hydroxyethyl) disulfide (1.62g, 10.50mmol), TEA (2.40g, 24.00mmol) and purified THF (10.00mL) was added to a 100mL one-neck round flask dropwise under ice propynyloxy oxalyl chloride was added (3.00g, 20.47mmol), was added dropwise at room temperature after the reaction 10H, to give a colorless transparent liquid, i.e., a compound of formula I (6.22g, 81% yield).
[0067] 2, under nitrogen, 5- (2-hydroxyethoxy) -2-nitrobenzyl alcohol (2.50g, 11.68mmol), TEA (6.30g, 62.26mmol) and THF (80.00mL) was added to 250mL three-ne...
Embodiment 3
[0074]
[0075] 1 Oxalyl chloride (25.00mL, 291.34mmol) and anhydrous dichloromethane (DCM, 60.00mL) was added to a 100mL one-neck round flask, 2-propyn-1-ol was added dropwise under ice-cooling (10.00 g, 178.57mmol), was added dropwise at room temperature the reaction after 3h. After reaction, the reaction solution was distilled off under reduced pressure at 98 ℃, to give a colorless transparent oily propynyloxy oxalyl chloride (17.32g, yield 65%).
[0076] Bis (2-hydroxyethyl) disulfide (1.84g, 11.93mmol), TEA (2.40g, 24.00mmol) and purified THF (10.00mL) was added to a 100mL one-neck round flask dropwise under ice propynyloxy oxalyl chloride was added (5.00g, 34.12mmol), was added dropwise at room temperature after the reaction 13H, to give a colorless transparent liquid, i.e., a compound of formula I (6.17g, 80% yield).
[0077] 2, under nitrogen, 5- (2-hydroxyethoxy) -2-nitrobenzyl alcohol (2.50g, 11.68mmol), TEA (4.00g, 39.53mmol) and THF (80.00mL) was added to 250mL three...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com