Process for synthesizing sodium nitrophenolate by continuously hydrolyzing nitrochlorobenzene
A technology of nitrochlorobenzene and sodium nitrophenolate, which is applied in the field of continuous hydrolysis of nitrochlorobenzene to synthesize sodium nitrophenolate, which can solve the problem of uneven quality of the output from the reactor, difficulty in automatic operation, and small scale of traditional processes and other issues, to achieve the effect of high conversion rate, reduced production cost and high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
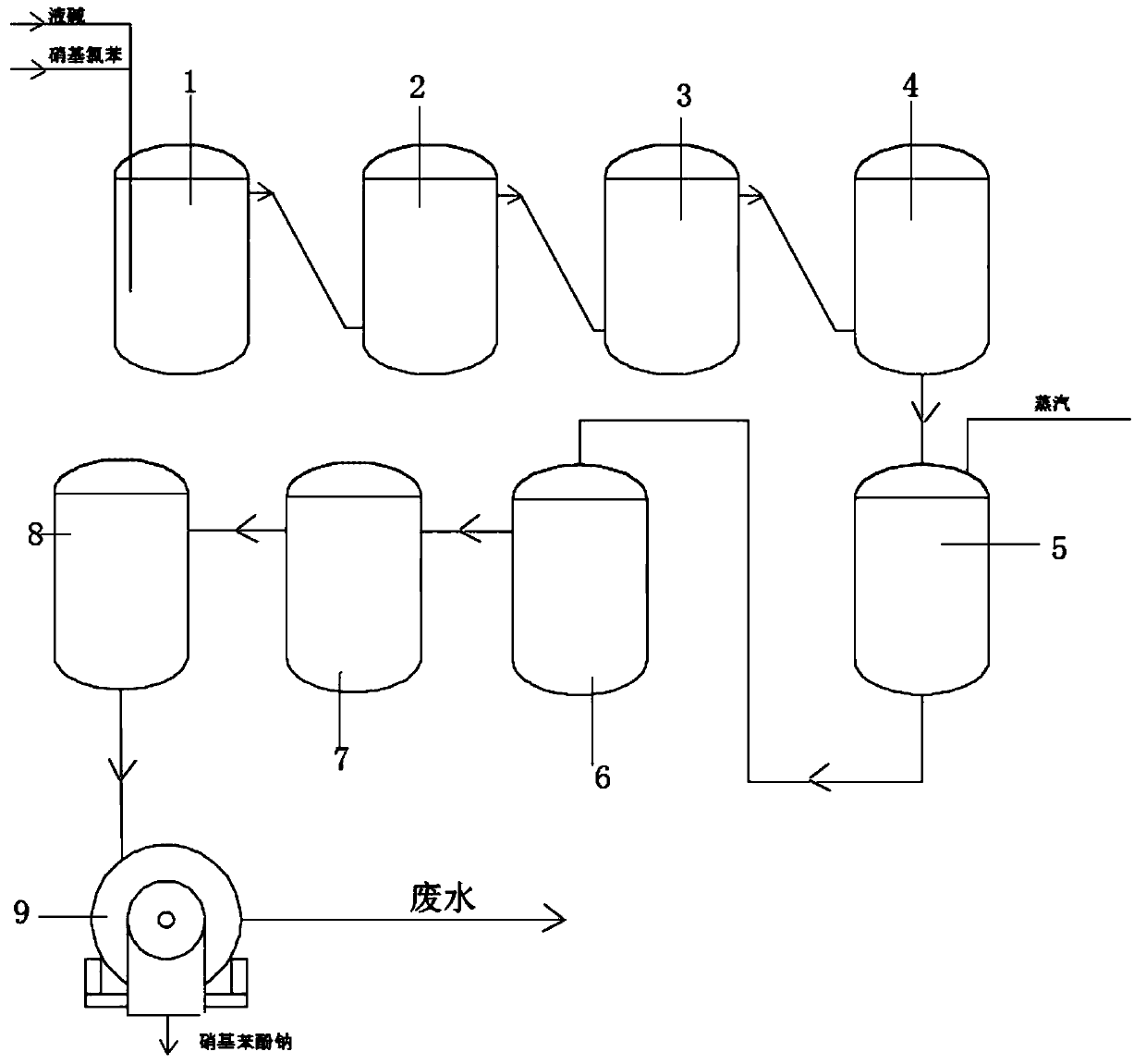
[0032] Step 1: Preheat 6% liquid caustic soda to 110°C, pump it and nitrochlorobenzene into hydrolysis kettle A respectively with a metering pump, and stir. The molar ratio of nitrochlorobenzene and liquid caustic soda is controlled at 1:2 to ensure that the nitrochlorobenzene The conversion of base chlorobenzene is complete;
[0033] Step 2: Heat the hydrolysis kettle A, and the temperature rises to 150°C. Since the reaction is an exothermic reaction, after overtemperature and overpressure, there is an exhaust pipeline, a safety valve and a high-pressure cooling water pipe to remove excess heat from the hydrolysis kettle A, so that The temperature in the hydrolysis tank A is kept constant at 15°C, and the pressure is controlled at 0.5-0.85Mpa;
[0034] Step 3: When the hydrolysis kettle A is almost full, preheat the temperature of the hydrolysis kettle B to 150°C and feed the material to the hydrolysis kettle B. When the feed liquid contacts the stirring paddle in the hydroly...
Embodiment 2
[0039] Embodiment two: step one: 10% liquid caustic soda is preheated to 120 ℃, and nitrochlorobenzene is pumped into the hydrolysis kettle A respectively with a metering pump, and stirred, and the molar ratio of nitrochlorobenzene and liquid caustic soda is controlled at 1: 3. Ensure that the conversion of nitrochlorobenzene is complete;
[0040] Step 2: Heat the hydrolysis kettle A, and the temperature rises to 160°C. Since the reaction is an exothermic reaction, after overtemperature and overpressure, there is an exhaust pipeline, a safety valve and a high-pressure cooling water pipe to remove excess heat from the hydrolysis kettle A, so that The temperature in the hydrolysis tank A is kept constant at 160°C, and the pressure is controlled at 0.5-0.85Mpa;
[0041] Step 3: When the hydrolysis kettle A is almost full, preheat the temperature of the hydrolysis kettle B to 160°C and feed the material to the hydrolysis kettle B. When the feed liquid contacts the stirring paddle ...
Embodiment 3
[0047] Step 1: Preheat 15% liquid caustic soda to 130°C, pump it and nitrochlorobenzene into hydrolysis kettle A respectively with a metering pump, and stir. The molar ratio of nitrochlorobenzene and liquid caustic soda is controlled at 1:5 to ensure that the nitrochlorobenzene The conversion of base chlorobenzene is complete;
[0048] Step 2: Heat the hydrolysis kettle A, and the temperature rises to 180°C. Since the reaction is an exothermic reaction, after overtemperature and overpressure, there is an exhaust pipeline, a safety valve and a high-pressure cooling water pipe to remove excess heat from the hydrolysis kettle A, so that The temperature in the hydrolysis tank A is kept constant at 175°C, and the pressure is controlled at 0.5-0.85Mpa;
[0049] Step 3: When the hydrolysis kettle A is almost full, preheat the temperature of the hydrolysis kettle B to 180°C and feed the material to the hydrolysis kettle B. When the feed liquid contacts the stirring paddle in the hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com