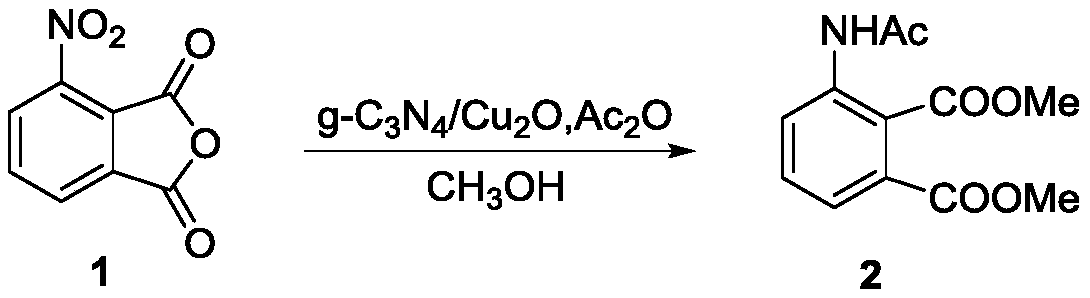Method for preparing dimethyl acetamidophthalate
A technology of dimethyl acetamidophthalate and nitrophthalic anhydride, which is applied in the field of organic synthesis, can solve problems such as low synthesis efficiency and lengthy steps, and achieves improved production efficiency and the formation of acetic acid p-acetamide. The effect of promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Put urea (50g) in an alumina crucible and put it into a muffle furnace, heat it up to 550°C at a heating rate of 5°C / min, keep it warm for 5h, and then cool it down to room temperature naturally, then place it in a dilute nitric acid solution (0.1 mol / L, 200mL) after stirring for 5min, the precipitate was filtered and dried at 80°C to obtain g-C 3 N 4 ;
[0017] (2) Dissolve copper acetate monohydrate (1mmol) in ethanol (50mL), heat up to 50°C, add glucose solution (0.2mol / L, 50mL) and sodium hydroxide solution (0.3mol / L, 50mL) and the g-C prepared in step (1) 3 N 4 (take 2.0g), after reacting for 15min, stop heating and stirring, after naturally cooling to room temperature, filter, precipitate is washed with deionized water, dry to obtain graphite phase carbon nitride supported cuprous catalyst (g-C 3 N 4 / Cu 2 O, hereinafter referred to as product A).
Embodiment 2
[0019] (1) Put urea (50g) in an alumina crucible and put it into a muffle furnace, heat it up to 600°C at a heating rate of 10°C / min, keep it warm for 3 hours, and then cool it down to room temperature naturally, then place it in a dilute nitric acid solution (0.2 mol / L, 200mL) after stirring for 10min, the precipitate was filtered and dried at 60°C to obtain g-C 3 N 4 ;
[0020] (2) Disperse copper sulfate pentahydrate (1mmol) in ethanol (100mL), heat up to 60°C, add glucose solution (0.1mol / L, 100mL) and sodium hydroxide solution (0.15mol / L, 100mL) under stirring ) and the g-C prepared in step (1) 3 N 4 (take 2.5g), after reacting for 10min, stop heating and stirring, after natural cooling to room temperature, filter, precipitate is washed with deionized water, dry to obtain graphite phase carbon nitride supported cuprous catalyst (g-C 3 N 4 / Cu 2 O, hereinafter referred to as product B).
Embodiment 3
[0022]
[0023] Weigh compound 1 (1mmol) and dissolve it in methanol (12mL), add 200mg of product A and acetic anhydride (2.0mmol) at room temperature, stir and react for 12 hours, filter, and after the filtrate is concentrated under reduced pressure, it is purified by silica gel column chromatography (EtOAc / petroleum ether=1 / 20 to 1 / 15) to obtain compound 2 (217mg, 86.4%), and the structural confirmation data of compound 2 are as follows:
[0024] Compound 2: m.p.104-105°C; ESIMS m / z 252.1[M+H] + ;Elemental AnalysisC 12 h 13 NO 5 : (theoretical value C, 57.37; H, 5.22, N, 5.58) measured value C, 57.60; H, 5.26; N, 5.65; 1 H NMR (400MHz, DMSO-d 6 )δ H 10.16(s,1H,N H Ac),7.82-7.65(m,2H,Ph-H),7.58-7.50(m,1H,Ph-H),3.80(s,3H,COOMe),3.78(s,3H,COOMe),2.21(s ,3H,COC H 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com