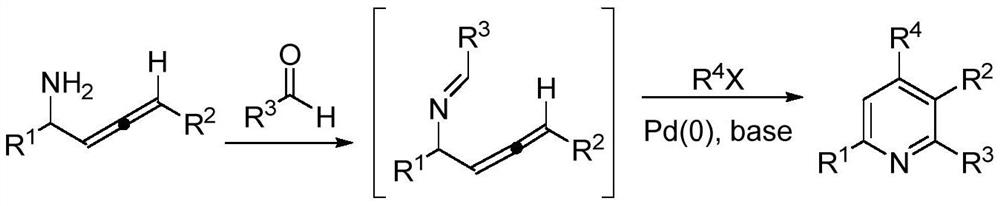
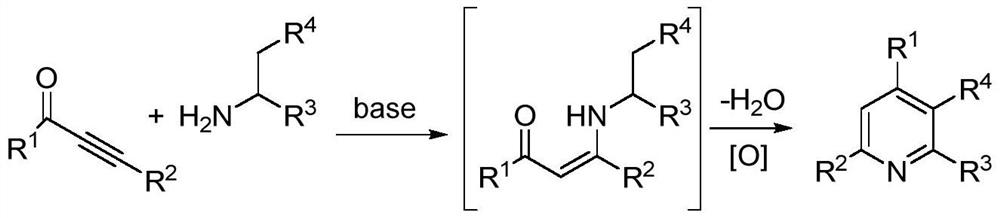
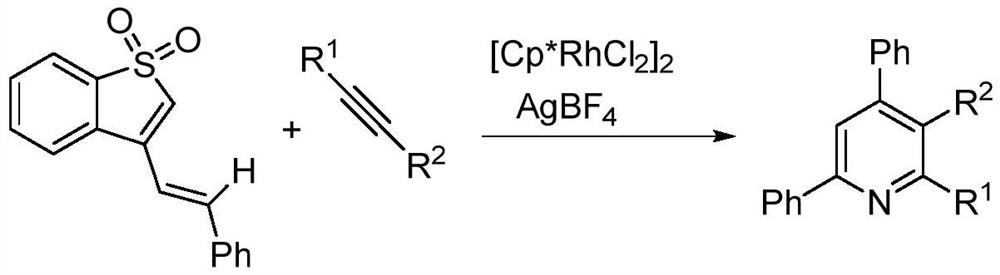
A kind of preparation method of 2,3,4,6-tetrasubstituted pyridine compounds
A ketone compound and compound technology are applied in the field of preparation of 2,3,4,6-tetra-substituted pyridine compounds, which can solve the problems of poor applicability of functional groups of substrates, achieve diverse structures, good application value, and avoid heavy metal pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of 2,3,4,6-tetraphenylpyridine (Ia)
[0053]
[0054] Acetophenone (1.2g, 10mmol), benzaldehyde (1.06g, 10mmol), diacetophenone (1.96g, 10mmol), ammonium acetate (2.31g, 30mmol) were stirred and reacted at 100°C for 20 hours. After the reaction, add water (15mL) to quench, extract with ethyl acetate (3×8mL), combine the organic phases and dry with anhydrous sodium sulfate, filter and spin the filtrate to dry the solvent under reduced pressure, and quickly separate by silica gel column chromatography The desired 2,3,4,6-tetraphenylpyridine (Ia) was obtained as 3.3 g of white solid, and the yield was 87% based on acetophenone.
[0055] 1 H NMR (400MHz, CDCl 3 )δ8.18-8.15(m,2H),7.78(s,1H),7.49(t,J=7.2Hz,2H),7.44-7.37(m,3H),7.24-7.19(m,6H),7.14 -7.12(m,2H),7.09-7.04(m3H),6.93-6.91(m,2H). 13 C NMR (101MHz, CDCl 3 )δ157.99,154.61,150.64,140.99,139.85,139.10,137.87,132.88,131.44,130.20,129.34,129.01,128.71,127.94,127.72,127.53,127.38,127.3...
Embodiment 2
[0056] Embodiment 2: Preparation of 6-(2-bromophenyl)-2,3,4-triphenylpyridine (Ib)
[0057]
[0058] 2-Bromoacetophenone (1.99g, 10mmol), benzaldehyde (1.06g, 10mmol), diacetophenone (1.96g, 10mmol), ammonium acetate (2.31g, 30mmol) were stirred at 120°C for 19 hours . After the reaction, add water (15mL) to quench, extract with ethyl acetate (3×8mL), combine the organic phases and dry with anhydrous sodium sulfate, filter and spin the filtrate to dry the solvent under reduced pressure, and quickly separate by silica gel column chromatography The desired 6-(2-bromophenyl)-2,3,4-triphenylpyridine (Ib) was obtained as 3.7 g of white solid, and the yield was 81% based on acetophenone.
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.77(dd,J=7.6,1.7Hz,1H),7.73–7.68(m,2H),7.46–7.41(m,1H),7.37–7.31(m,2H),7.28(d,J= 1.7Hz,1H),7.24–7.16(m,6H),7.16–7.12(m,2H),7.12–7.05(m,3H),6.98–6.92(m,2H). 13 C NMR (101MHz, CDCl 3 )δ158.03,156.61,156.58,149.64,140.97,140.70,139.43,137.61,133.45,133.04,13...
Embodiment 3
[0060] Example 3: Preparation of 6-(2-chlorophenyl)-2,3,4-triphenylpyridine (Ic)
[0061]
[0062] 2-Chloroacetophenone (1.54g, 10mmol), benzaldehyde (1.06g, 10mmol), diacetophenone (1.96g, 10mmol), ammonium acetate (2.31g, 30mmol) were stirred and reacted at 110°C for 18 hours . After the reaction, add water (15mL) to quench, extract with ethyl acetate (3×8mL), combine the organic phases and dry with anhydrous sodium sulfate, filter and spin the filtrate to dry the solvent under reduced pressure, and quickly separate by silica gel column chromatography The desired 6-(2-chlorophenyl)-2,3,4-triphenylpyridine (Ic) was obtained as 3.5 g of white solid, and the yield was 83% based on acetophenone.
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.82(dd, J=7.6,1.8Hz,1H),7.74(s,1H),7.50(dd,J=7.8,1.4Hz,1H),7.39(td,J=7.5,1.5Hz,1H ),7.36-7.32(m,3H),7.23–7.16(m,6H),7.15–7.05(m,5H),6.96–6.92(m,2H). 13 C NMR (101MHz, CDCl 3)δ158.15,155.16,149.67,140.72,139.44,138.99,137.61,133.04,132.38,131.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com