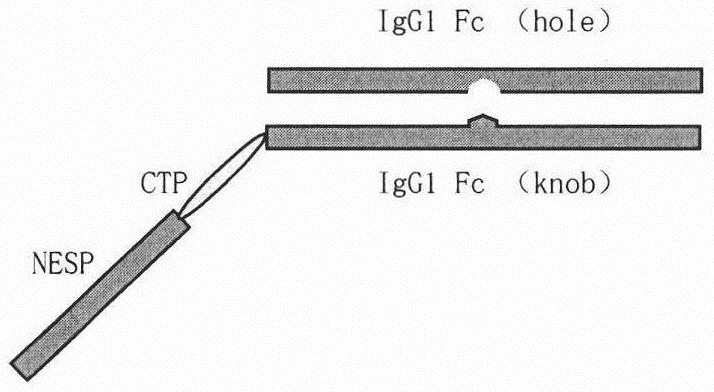
A long-acting fusion protein with erythropoietin activity
A technology of erythropoietin and fusion protein, which is applied to the preparation of the above compounds, recombinant proteins, and long-acting fusion proteins, and can solve problems such as changing EPO and decreasing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Construction of a vector encoding NESP-CTP-IgG1Fc:IgG1Fc' fusion protein.
[0041]The nucleic acid sequences encoding the first strand and the second strand were artificially synthesized by Nanjing GenScript Company and respectively connected to the PUC57 vector, named pUC57nesp and PUC57Fc respectively. Double-digest PUC57nesp with AvrII and Bstz17I, double-digest PUC57Fc with EcoRV and PacI, and double-digest Invitrogen pCHO1.0 expression vector with EcoRV and PacI at the same time, recover the gene fragments from the gel, and recover the digested vector with a DNA purification and recovery kit pCHO1.0, and two gene fragments nesp and Fc.
[0042] The vector pCHO1.0 recovered by enzyme digestion was connected to Fc, and the correctly connected plasmid was named pCHO-Fc, and pCHO-Fc was digested with AvrII and Bstz17I, and the DNA purification and recovery kit was directly recovered, and the same double The nesp gene connection recovered by enzyme digestion...
Embodiment 2
[0043] Example 2. Preparation and cell culture of cell lines expressing NESP-CTP-IgG1Fc: IgG1Fc' fusion protein.
[0044] The recombinant expression vector pCHO-NESP-Fc was transformed into Escherichia coli DH5α competent cells, the transformed bacteria were rejuvenated with LB liquid screening medium containing kana and then expanded to 100ml for mass culture, shaken at 37°C overnight, and the plasmid was extracted and purified. The final plasmid DNA concentration was adjusted to 1 μg / μL for electroporation to transform CHO-S cells.
[0045] One day before transfection, the host cells were divided into 0.5×10 6 The seeding density was subcultured to 20 mL of fresh medium. Cells in the logarithmic growth phase were collected in parallel on the day of electroporation, and the number of cells in each group was 1×10 7 After centrifuging to remove the medium, wash the cells with PBS, discard the supernatant, resuspend the cells in 0.8ml PBS and add them to the electroporation cu...
Embodiment 3
[0048] Example 3. Purification of fusion proteins
[0049] The solid-liquid separation is carried out by conventional centrifugation technology, and the cell components in the fermentation system are removed. The fermentation supernatant was purified using the following steps:
[0050] A purification method for purifying an Fc fusion protein according to this embodiment specifically includes the following steps:
[0051] 1) First use Mabselect SuRe LX to capture the fusion protein, including: equilibrate the chromatographic column for 5 column volumes with affinity balance solution (25mM Tris, 25mM NaCl, pH7.5); load the sample, and the retention time of the sample is 10min; complete the loading After sampling, rebalance 4 column volumes with affinity balance solution, then wash 4 column volumes with eluent (25mM Tris, 0.3M NaCl, pH7.5); wash the layer with 50mM acetate, pH4.6 The column was analyzed with 3 column volumes, the sample was eluted with 50mM acetate, pH=3.5, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com