Nucleic acid aptamer specifically recognizing bovine pregnancy-associated glycoprotein 4 and its application
A nucleic acid aptamer and glycoprotein technology, which can be applied in the fields of instruments, biochemical equipment and methods, analytical materials, etc., and can solve problems such as no research reports on livestock pregnancy-related glycoprotein nucleic acid aptamers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Screening of bPAG4 nucleic acid aptamers
[0033] 1. Synthetic random single-stranded DNA (ssDNA) library and primers:
[0034] Random single-stranded DNA (ssDNA) library:
[0035] 5'-CTACGGTGCCTTGAAGTGAC-N36-CATAGCAGGTCACTTCCAGG-3', wherein, N36 represents 36 random nucleotides, and the library was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.;
[0036] Upstream primer: 5'-FAM-CTACGGTGCCTTGAAGTGAC-3',
[0037] Downstream primer: 5'- 20A-spacer18-CCTGGAAGTGACCTGCTATG-3',
[0038] Among them, in the downstream primers, 20A represents a polyA tail composed of 20 adenosine (A), and Spacer 18 represents an 18-atom hexaethylene glycol interarm. The above primers were synthesized by Nanjing GenScript Biotechnology Co., Ltd.
[0039] Use DPBS buffer (NaCl: 8 g / L, KCl: 0.2 g / L, Na2HPO4: 1.15 g / L, KH2PH4: 0.2 g / L; PH 7.4) and stored at -20°C for later use.
[0040] 2. Magnetic beads-bPAG4 protein (MB-bPAG4) coupling
[0041] Take 50 μL of carboxylate...
Embodiment 2
[0053] Embodiment 2, the affinity analysis of nucleic acid aptamer
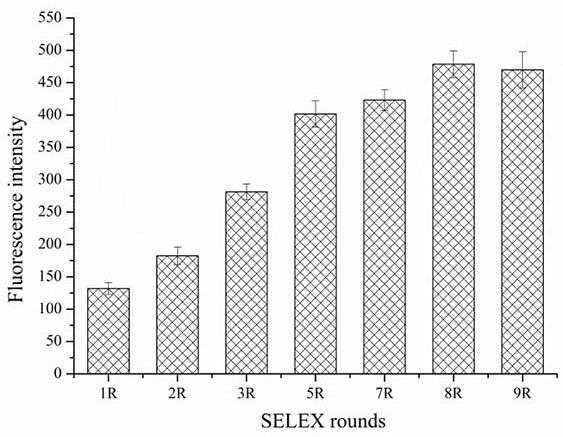
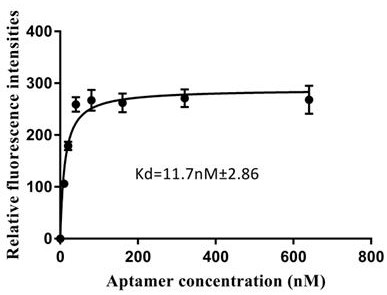
[0054] Several nucleic acid aptamers synthesized in Example 1 were taken, and each nucleic acid aptamer was prepared into a series of gradient concentrations (0nM, 100 nM, 200 nM, 400 nM, 800 nM, 1600 nM, 3200 nM, 6400 nM in 10 mM PBS nM) aptamer solution; according to step 4, analyze the binding of each nucleic acid aptamer to MB-bPAG4 in turn, use GraphPadPrism 7.0 software to fit the binding curve, and calculate the dissociation constant Kd value of each nucleic acid aptamer. image 3 It is the saturated binding curve of the nucleic acid aptamer shown in SEQ ID No.1 and the target protein bPAG4, the aptamer has high affinity with the bPAG4 protein, and the dissociation constant is 11.7 nM. The secondary structure prediction analysis of the aptamer sequence shown in SEQ ID No. 1 was carried out using online Mfold, and the results are shown in Figure 4 .
Embodiment 3
[0055] Embodiment 3: the specificity analysis of nucleic acid aptamer
[0056] Prepare MB-bPAG1, MB-bPAG4, MB-bPAG9, MB-BSA and MB-OVA respectively according to step 2, dilute the nucleic acid aptamer sequence shown in SEQ ID No.1 with 10mM PBS to a concentration of 0.5 μM, follow the steps 4 Analyze the binding of the nucleic acid aptamer to each protein-coupled magnetic bead, and the magnetic bead not connected to any protein is used as a negative control. See the test results Figure 5 , the nucleic acid aptamer shown in SEQ ID No.1 preferentially binds to the target protein bPAG4, and has a higher binding ability with similar family proteins, but does not bind with other BSA and OVA proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com