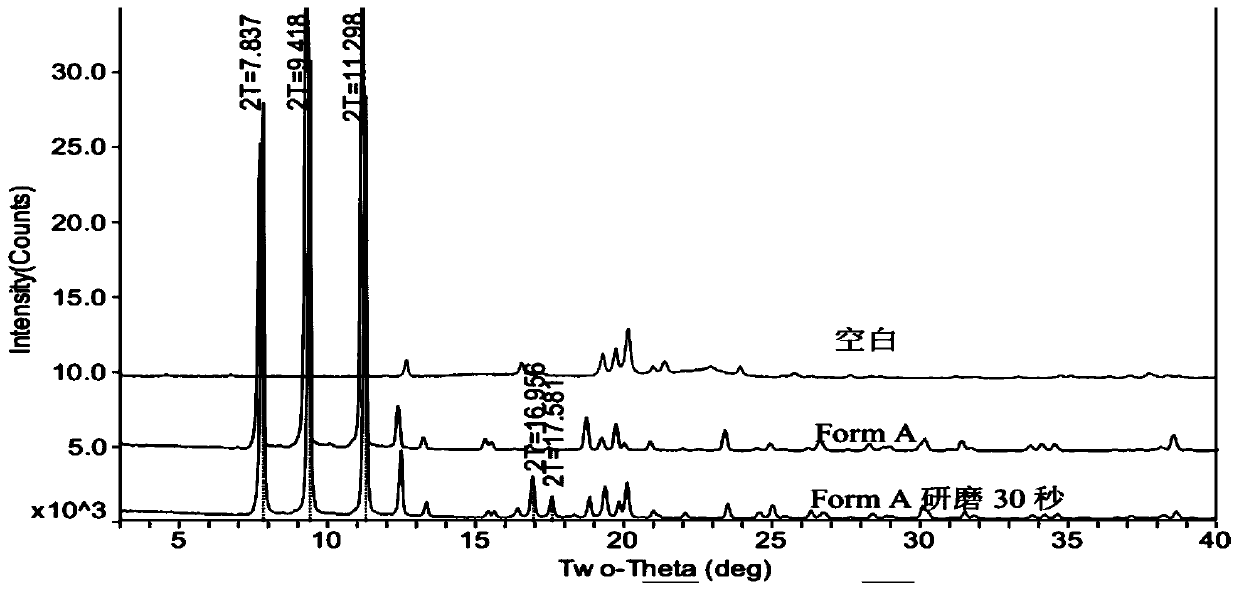
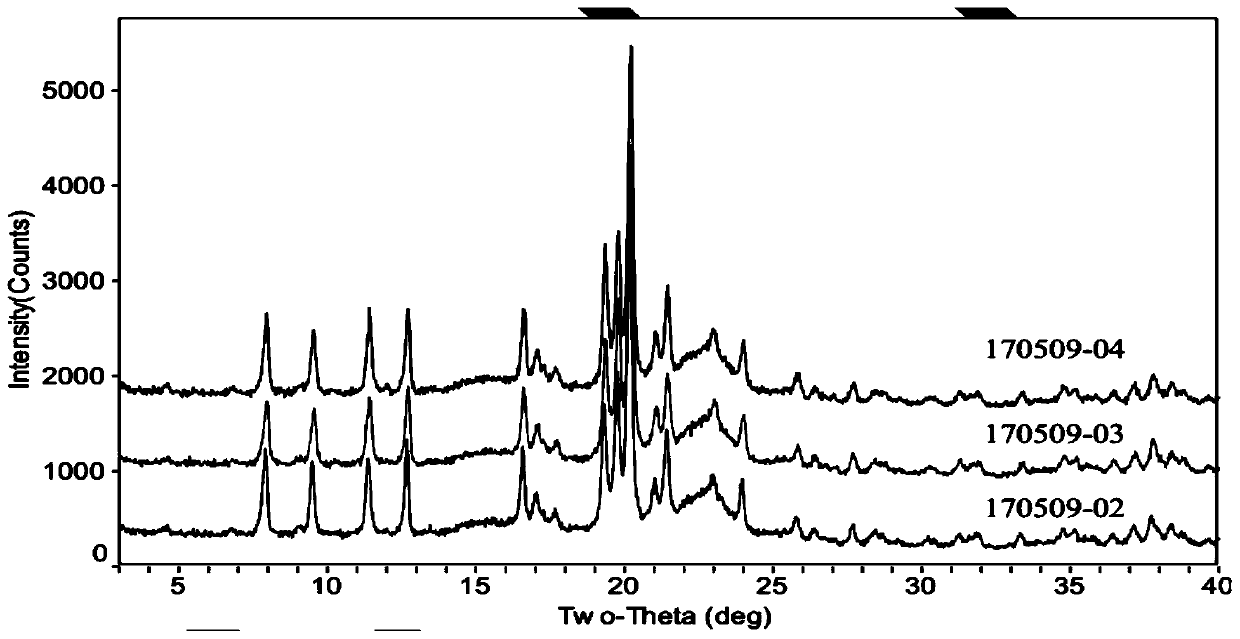
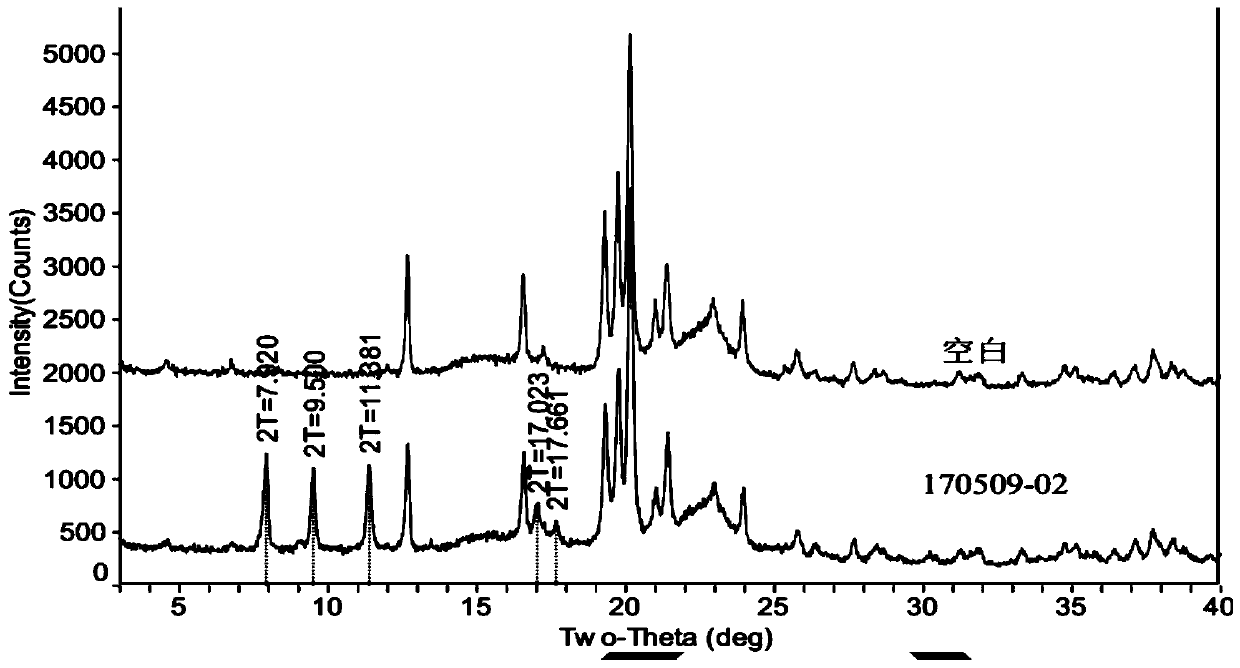
Method for detecting dihydroartemisinin crystal form in dihydroartemisinin tablet
A technology of dihydroartemisinin tablets and dihydroartemisinin, which is applied in the field of medicine, can solve the problems of unreported crystal forms of dihydroartemisinin, and achieve the effects of good durability, precision and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of Dihydroartemisinin Tablets (170509-02)
[0026] Take the raw and auxiliary materials of prescription quantity respectively, make dihydroartemisinin contain 18%, lactose contain 52%, microcrystalline cellulose contain 18%, sodium carboxymethyl starch contains 8%, polyvinylpyrrolidone contains 3.7%, stearin Magnesium acid contains 0.3%. Lactose, microcrystalline cellulose, sodium carboxymethyl starch, and polyvinylpyrrolidone are passed through a 80-mesh sieve. Add the above-mentioned raw and auxiliary materials into the hopper of the mixer for mixing, and perform tablet compression after mixing.
Embodiment 2
[0027] Example 2: Preparation of Dihydroartemisinin Tablets (170509-03)
[0028] Take the raw and auxiliary materials of prescription quantity respectively, make dihydroartemisinin contain 10%, lactose contain 40%, microcrystalline cellulose contain 30%, sodium carboxymethyl starch contains 15%, polyvinylpyrrolidone contains 4.5%, stearin Magnesium acid contains 0.5%. Lactose, microcrystalline cellulose, sodium carboxymethyl starch, and polyvinylpyrrolidone are passed through a 80-mesh sieve. Add the above-mentioned raw and auxiliary materials into the hopper of the mixer for mixing, and perform tablet compression after mixing.
Embodiment 3
[0029] Example 3: Preparation of Dihydroartemisinin Tablets (170509-04)
[0030] Take the raw and auxiliary materials of recipe quantity respectively, make dihydroartemisinin contain 15%, lactose contain 35%, microcrystalline cellulose contain 32%, carboxymethyl starch sodium contains 13%, polyvinylpyrrolidone contains 4.2%, stearin Magnesium acid contains 0.8%. Lactose, microcrystalline cellulose, sodium carboxymethyl starch, and polyvinylpyrrolidone are passed through a 80-mesh sieve. Add the above-mentioned raw and auxiliary materials into the hopper of the mixer for mixing, and perform tablet compression after mixing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com