Biodegradable polyesteramide used for the treatment of arthritic disorders
An arthritic, inflammatory technology in the field of biodegradable polyesteramides for the treatment of arthritic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Fabrication of TAA-loaded PEA-III-Bz microparticles
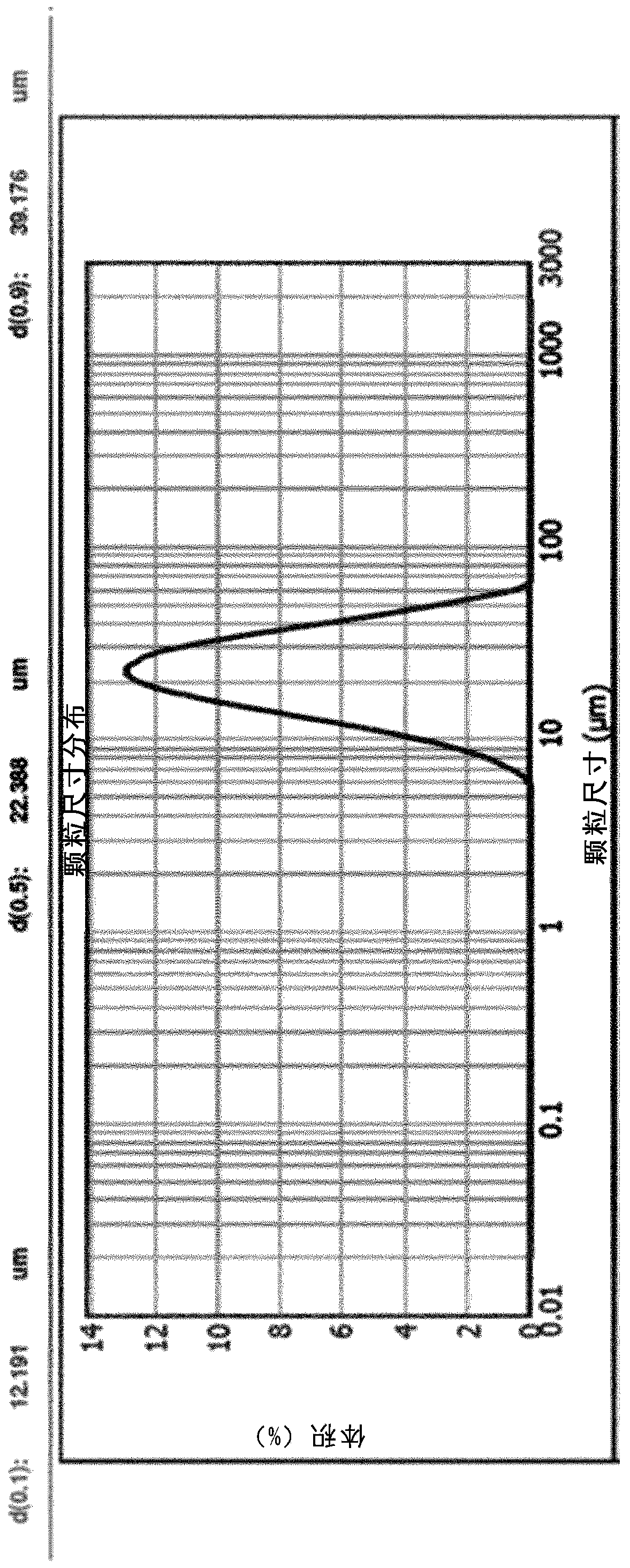
[0102] 300 mg of PEA-III-Bz was dissolved in dichloromethane. 75 mg of TAA was added to the solution and homogenized by sonication. use The suspension was added to 20 ml of cold water containing 1 wt% poly(vinyl alcohol) under high shear. After obtaining a stable suspension, the particles were hardened for 12 hours in 100 ml of water containing 1% by weight of poly(vinyl alcohol). Excess water and surfactant were removed by rinsing and centrifugation. Finally, the particles are frozen and dried under vacuum. exist figure 1 A picture of the particles is given in . figure 2 The particle size distribution is given in .
Embodiment 2
[0104] Culture of chondrocytes in the presence of TAA-loaded PEA-III-Bz microparticles
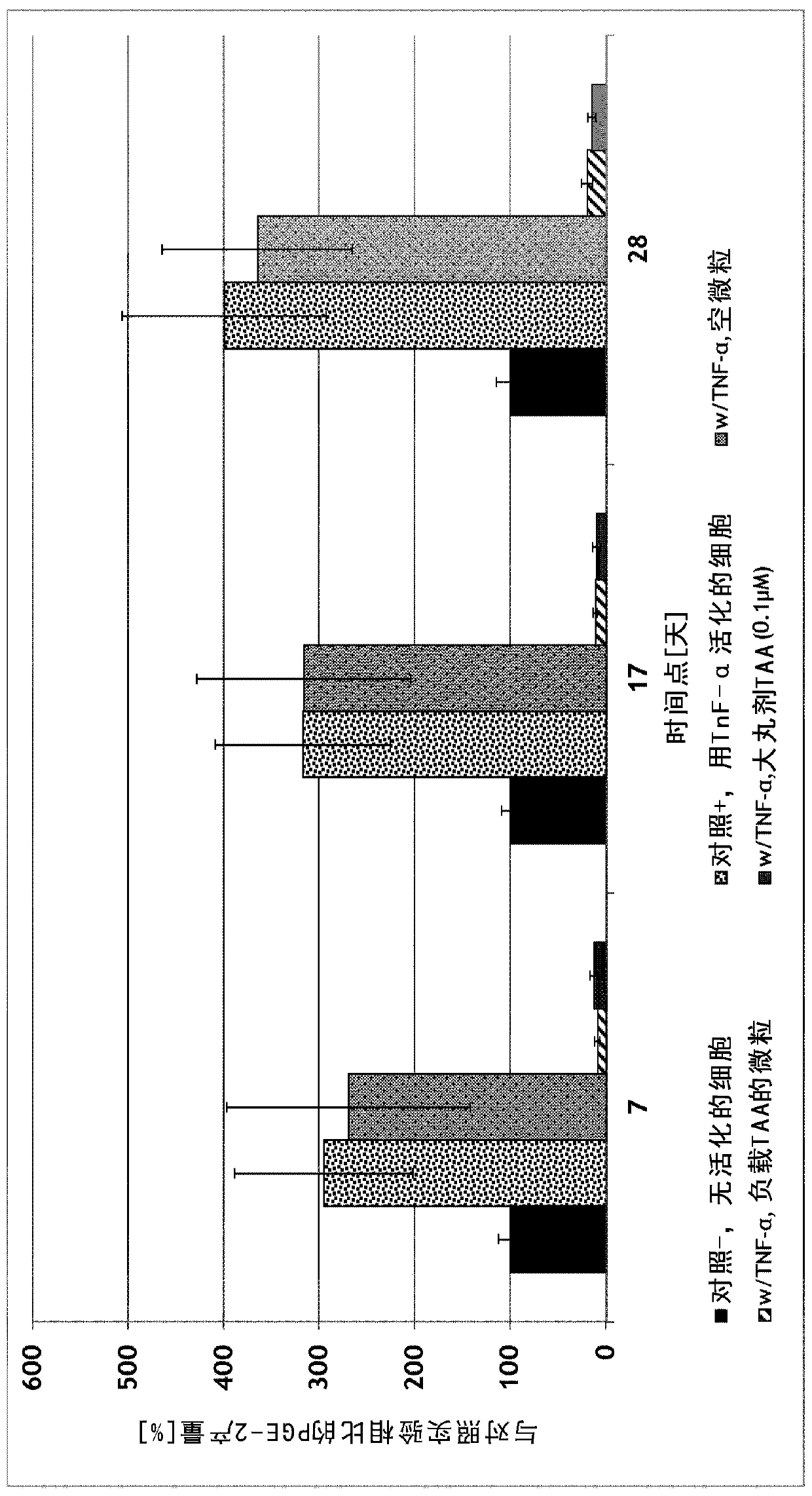
[0105] OA chondrocytes were harvested from three human donors and cultured from total knee arthroplasty. Cells were incubated with 5 µg of microparticles produced in Example 1 in transwells. Cells and media were collected every three days and transwells were transferred to wells with freshly plated OA chondrocytes from the same donor. The amount of PGE-2 (prostaglandin-E2) produced by the cells during the incubation time was determined by ELISA.
[0106] The total duration of the experiment was 38 days.
[0107] Cells were cultured under the following conditions:
[0108] 1. Control experiment; no particles
[0109] 2. Positive control, chondrocytes stimulated by TNF-α, without granules
[0110] 3. Chondrocytes stimulated by TNF-α were incubated with 5 μg of empty PEA-III-Bz particles
[0111] 4. Chondrocytes stimulated by TNF-α were incubated with 5 μg of PEA-III-Bz particles load...
Embodiment 3
[0115] Cumulative release of fluorescein from PEA-III-Bz membranes
[0116] A proof-of-principle study has been performed employing fluorescein as a slowly released dye from the membrane.
[0117] In acute inflammation, polymorphonuclear neutrophils (PMNs) are the first type of cells to migrate to the site of inflammation, where they produce several pro-inflammatory mediators, including attracting other PMNs and other cell types (such as monocytogenes). Chemokine for nuclear cells - macrophages and lymphocytes), corresponding to chronic inflammation. Neutrophils and macrophages are two cell types known to interact with biomaterials, which may lead to their degradation. Using differentiated HL60 neutrophil-like cells, the interaction of neutrophil-like cells with biomaterials can be modeled. Neutrophils are inflammatory cell first responders that migrate to sites of inflammation and are known to have a high level of interaction with foreign bodies such as biological materia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com