Multivalence recombinant protein vaccine for chicken coccidiosis, and preparation method and application of multivalence recombinant protein vaccine for chicken coccidian
A technology of recombinant protein and chicken coccidia, which is applied in multivalent vaccines, immunoglobulins, immunoglobulins from serum, etc., can solve the problems of difficult popularization and application and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Expression and purification of embodiment 1 recombinant protein
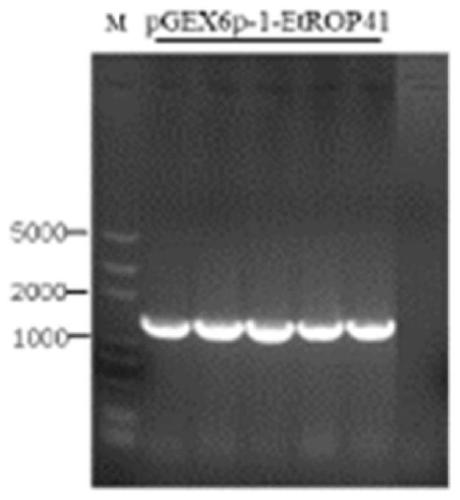
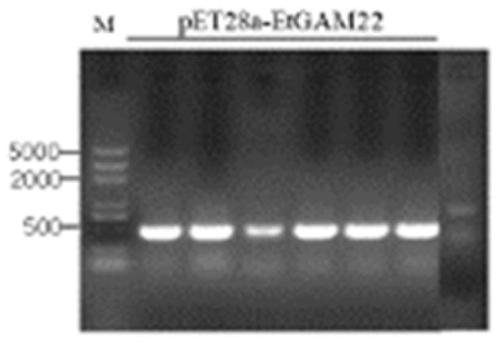
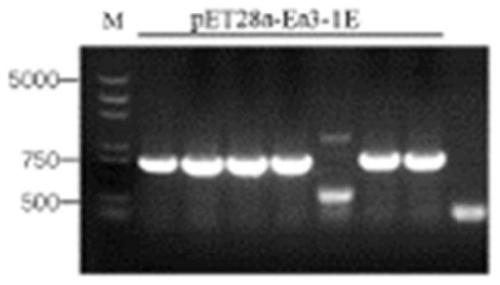
[0045] In the present invention, the antigen of Eimeria tenella is EtROP. The accession number in the ToxoDB database is ETH_00005405 (https: / / toxodb.org / toxo / app / record / gene / ETH_00005405), the length of the gene fragment encoding the protein The accession number of EtGAM22 in the ToxoDB database is ETH_00035480 (https: / / toxodb.org / toxo / app / record / gene / ETH_00035480), and the length of the gene fragment encoding the protein is 597bp; the accession number of EmGAM56 in the ToxoDB database The number is EMWEY_00026710 (https: / / toxodb.org / toxo / app / record / gene / EMWEY_00026710), and the length of the gene fragment encoding the protein is 1422bp; the accession number of Ea3-1E in the ToxoDB database is EAH_00057690 (https: / / toxodb.org / toxo / app / record / gene / EAH_00057690), the length of the gene fragment encoding the protein is 1737bp.
[0046] 1. Cloning of genes and construction of recombinant plasmids
[0047]...
Embodiment 3
[0085] Example 3 Evaluation of Maternal Immunization on the Protective Effect of Seven-day-old Offspring Chicks
[0086] 1. Preparation of sporulated oocysts of Eimeria gallinarum
[0087] Five 14-day-old coccidian-free chickens in each of the three groups were orally infected with 1×10 4 sporulated E.tenella sporulated oocysts, 2×10 5 sporulated oocysts of E.maxima and 1.5×10 5 Each group of sporulated oocysts of E.acervulina was reared in different isolators. After 144h, 121h and 97h, the feces of each group were collected for 3 consecutive days, and the oocysts were collected by floating in saturated saline (method omitted). The collected oocysts were cultured on a shaker at 27°C for sporulation. During this period, an appropriate amount of oocyst liquid was taken to examine the degree of sporulation under a microscope. When 95% of the oocysts were completely sporulated, they were stored at 4°C for later use.
[0088] 2. Evaluation of the protective effect of 7-day-old ...
Embodiment 4
[0148] Example 4 Evaluation of the protective effect of maternal immunization on 14-day-old offspring chicks
[0149] 1. The preparation method of fresh Eimeria chicken fresh oocyst: with embodiment 3;
[0150] 2. Evaluation of the protection effect of 14-day-old offspring chicks
[0151] 2.1 Experimental design
[0152] Eggs from the hen after three vaccinations were selected for incubation, and the eggs of the non-immunized hen were used for subsequent incubation in the challenge control group and the blank control group. The experiment was divided into 4 groups, with 10 14-day-old chicks in each group, randomly divided into groups, and the chicks with low or high body weight were eliminated. The first group of maternal immunization group (10 feathers), the second group of adjuvant immunization group (10 feathers), the third group of challenge control group (positive control group) (10 feathers), the fourth group of blank control group (negative control group). At the ag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com