A quick-acting and safe ticagrelor oral spray and preparation method thereof
A technology of oral spray and ticagrelor, which is applied in the field of ticagrelor oral spray and its preparation, to achieve the effect of stable dispersion system, good taste and stability, and convenient taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Effect of Particle Size of Ticagrelor API on Solubilization Effect
[0037] The raw material of ticagrelor was micronized using a jet mill, and the results are shown in Table 1. The measurement results showed that micronizing the raw material was helpful to improve the solubility of ticagrelor, but adding a solubilizer was still needed to further improve the solubility. where, * indicates D compared with the particle size of 20 μm 90 There was a significant difference in the solubility of ticagrelor at ≤10 μm.
[0038] Table 1 The influence of the particle size of raw materials on the solubilization effect of ticagrelor
[0039] API Particle Size solvent Solubility (mg / mL) D. 90 :20μm
Embodiment 2
[0040] Embodiment 2: prepare the product of different particle size, solubility
[0041] Table 2 Product Prescription Form
[0042]
[0043]
[0044] Preparation method: a. Add RC-591 microcrystalline cellulose-carboxymethyl cellulose sodium into purified water under stirring, and stir well until the swelling is complete; b. Micronize the raw material drug to a suitable particle size Adding a wetting agent and a transdermal absorption-promoting agent to the powder and fully stirring to obtain a stable and uniform raw material drug mixture; c. Adding the colloid solution obtained in step a to step b and fully stirring to obtain a stable and uniform dispersion system; d .Add taste regulator, pH regulator and other auxiliary materials to the uniform dispersion system obtained in step c, fully stir and adjust the pH to between 5.0 and 9.0; obtain the drug-containing solution, and fill the drug-containing solution, Promptly obtain described ticagrelor oral spray.
[0045] T...
Embodiment 3
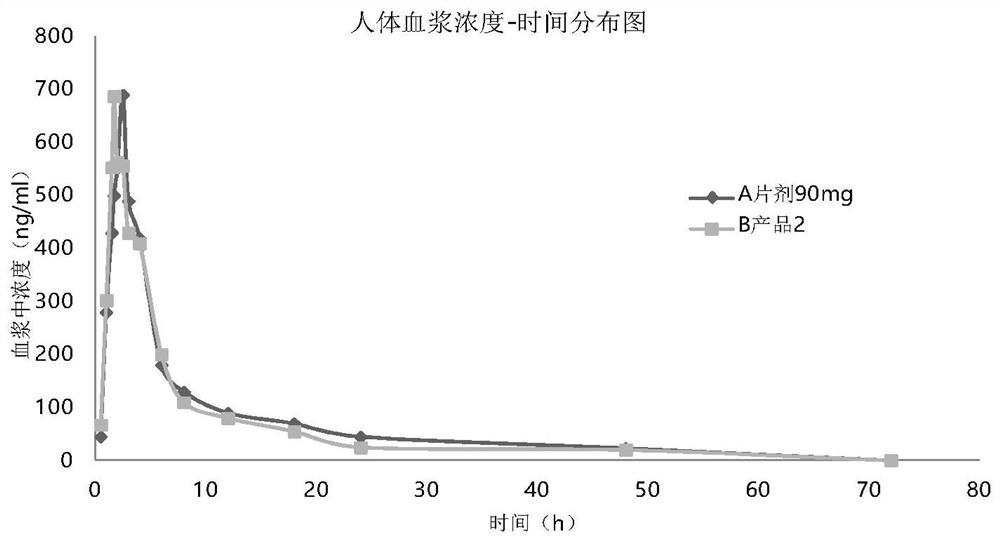
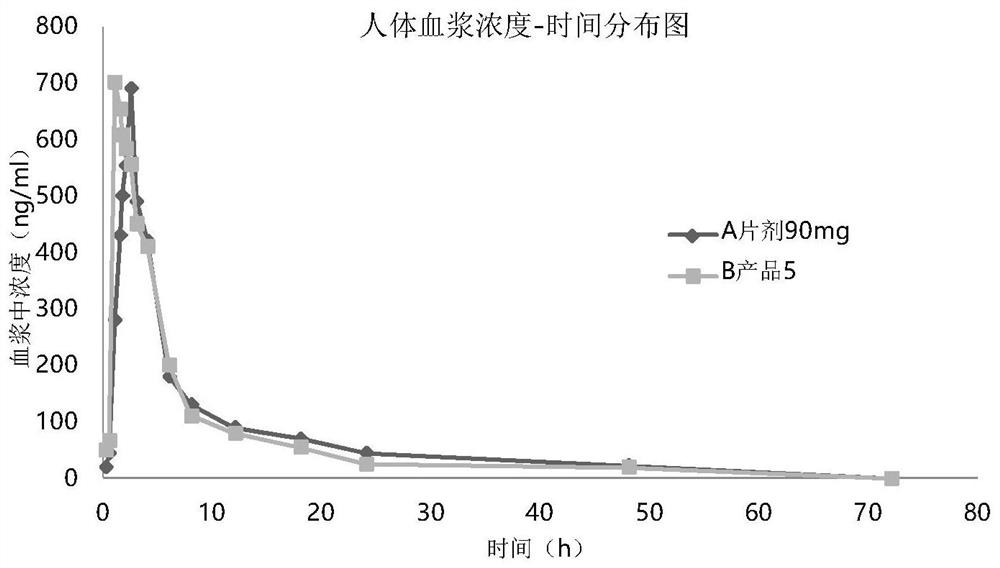
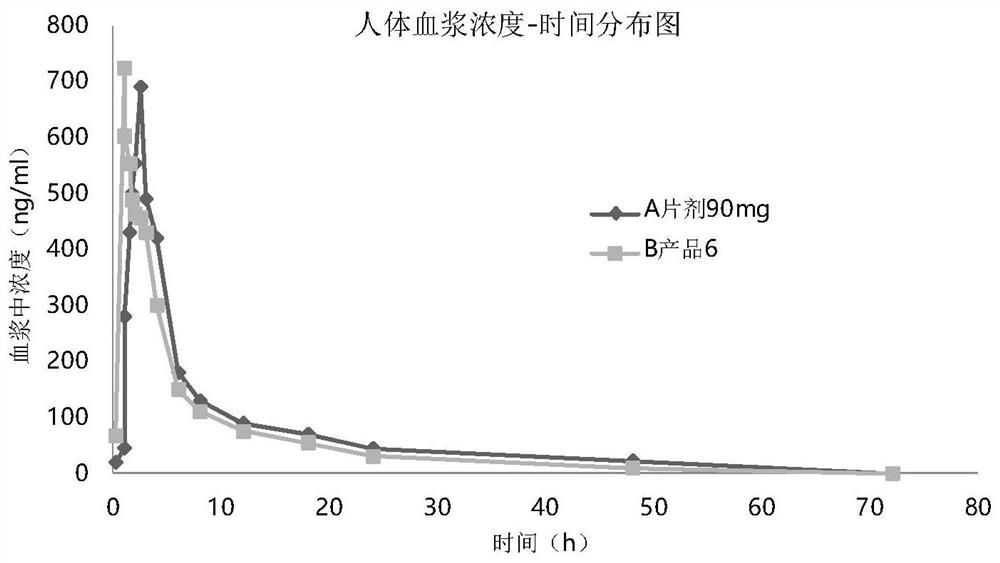
[0046] Embodiment 3: pharmacokinetic animal experiment
[0047] In vivo absorption experiments were carried out on the prepared seven products. Test method is as follows: get 45 beagle dogs fasting, be divided into 9 groups, every group of 5: one group is given normal saline gavage (negative control), one group is given commercially available tablet (trade name: ) orally (positive control), and the other seven groups were given seven product sprays in Table 3 respectively. Oral group given Tablet 90mg, experimental group oral administration spray 30mg / spray, totally 3 sprays. When spraying, fix the dog's head to keep the mouth level, spray the drug quantitatively into the mouth, and keep the dog's head in a horizontal position for more than 1 minute after administration to prevent the liquid from flowing out. There must be an appropriate interval after administration of each dosage form to ensure sufficient time for blood collection.
[0048] Before administration (0h) and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com