Cinnamic acid amide diazole derivatives and application thereof in antifungal drugs
A technology of cinnamic acid amidodiazoles and antifungal drugs, applied in antifungal agents, organic chemistry, etc., can solve the problems of narrow antibacterial spectrum, toxic and side effects, and interactions, and achieve good antifungal, novel structure, and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
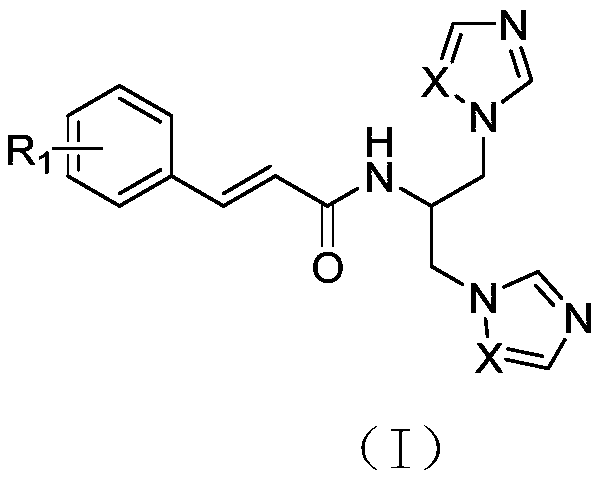
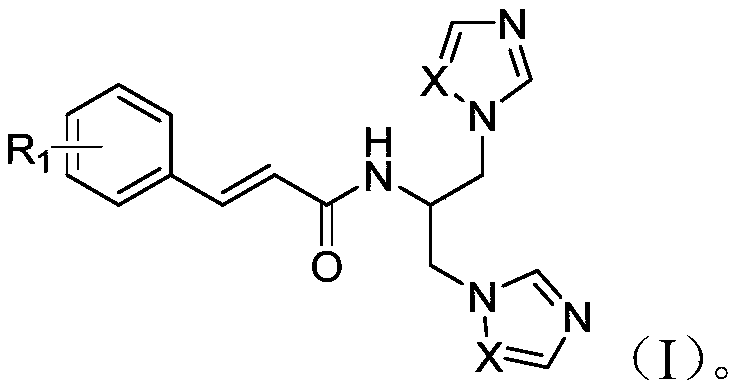
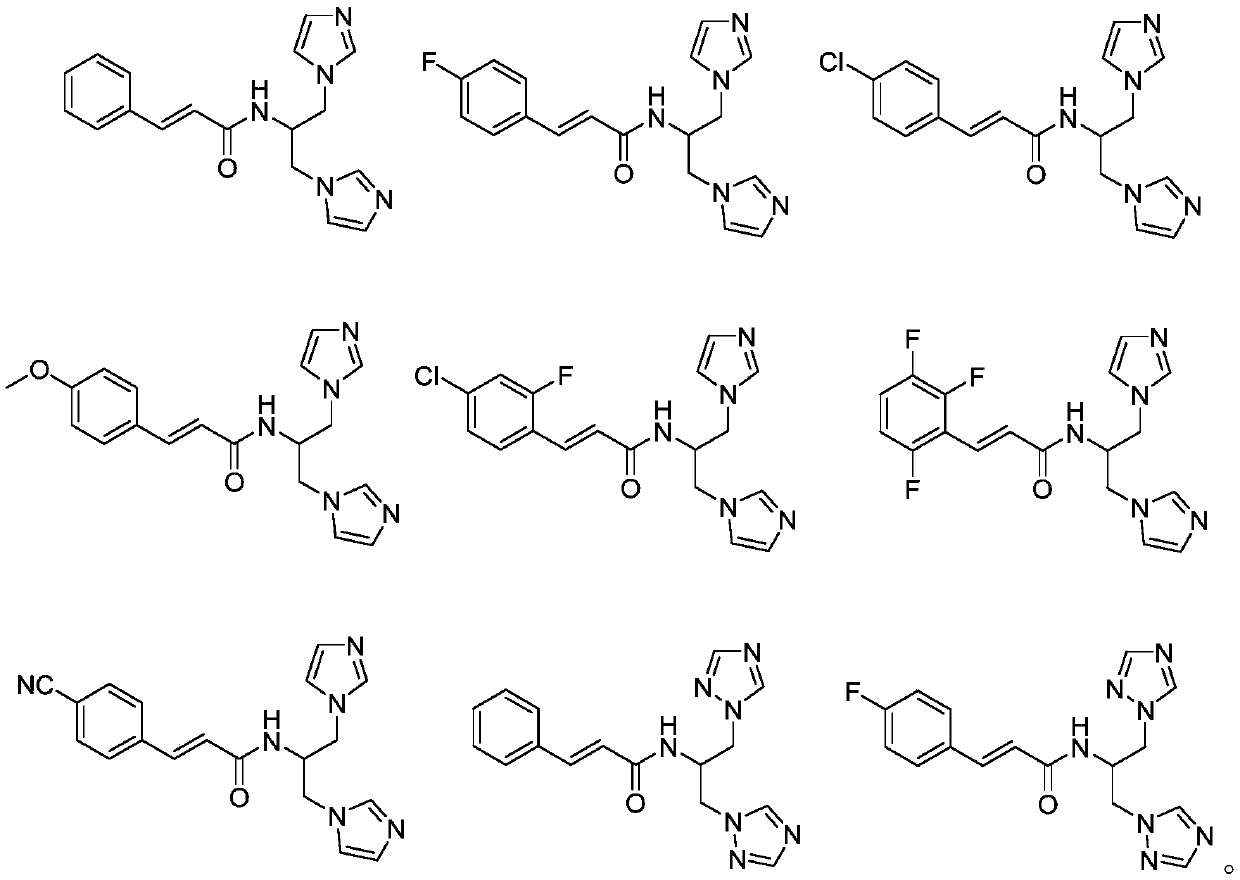
[0023] Example 1 N-[1,3-bis(1H-imidazol-1-yl)propan-2-yl]-cinnamamide.
[0024]
[0025] Step 1 Preparation of intermediate 3.
[0026] Cinnamic acid (3.0g, 20.25mmol), EDC·HCl (4.27g, 22.27mmol) and HOBt (3.01g, 22.27mmol) were dissolved in 50mL DMF, stirred at room temperature for 1h, then serinol (2.03g, 22.27mmol) and DIEA (6.54g, 50.6mmol), stirred at room temperature for 10h, TLC monitored the reaction was complete, added 100mL of water, extracted with ethyl acetate, washed the organic layer with saturated brine, Na 2 SO 4 Let dry overnight. The desiccant was filtered off, and concentrated under reduced pressure to obtain 3.82 g of light yellow oil, with a yield of 85.27%.
[0027] LC-MS m / z[M+H] + 222.2.
[0028] Step 2 Preparation of N-[1,3-bis(1H-imidazol-1-yl)propan-2-yl]-cinnamamide.
[0029] Intermediate 3 (1.00g, 4.52mmol), CDI 1.47g (9.04mmol) and imidazole 1.23g (18.08mmol) were dissolved in 30mL acetonitrile and reacted at 70°C for 7h. TLC monitors th...
Embodiment 2
[0032] Example 2 N-[1,3-bis(1H-imidazol-1-yl)propan-2-yl]-3-(4-fluorophenyl)-acrylamide.
[0033]
[0034] LC-MS m / z[M+H] + 339.2. 1 H-NMR (400MHz, DMSO-d 6 )δ9.30(d,J=8.1Hz,1H),7.85(d,J=15.2Hz,1H),7.72(d,J=7.4Hz,2H),7.63(s,2H),7.42(d, J=7.5Hz, 2H), 7.21(s, 2H), 6.85(s, 2H), 6.35(d, J=15.2Hz, 1H), 3.94(d, J=7.1 Hz, 4H), 3.69-3.60( m, 1H).
Embodiment 3
[0035] Example 3 N-[1,3-bis(1H-imidazol-1-yl)propan-2-yl]-3-(4-chlorophenyl)-acrylamide.
[0036]
[0037] LC-MS m / z[M+H] + 356.2. 1 H-NMR (400MHz, DMSO-d 6 )δ9.31(d, J=8.2Hz, 1H), 7.85(d, J=15.2Hz, 1H), 7.70(d, J=7.5Hz, 2H), 7.63-7.60(m, 4H), 7.42( d,J=7.5Hz,2H),7.22(s,2H),6.84(s,2H),6.32(d,J=15.1Hz,1H),3.93 (d,J=7.2Hz,4H),3.68- 3.61(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com