Diterpenoid compound in fir and preparation method and application thereof in pharmacy
A technology of compounds and diterpenoids, applied in the separation/purification of carboxylic acid compounds, separation/purification of hydroxyl compounds, separation/purification of carbonyl compounds, etc., can solve the diterpenoid components and pharmacological activities, and there is no report And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of diterpenoids derived from Douglas fir
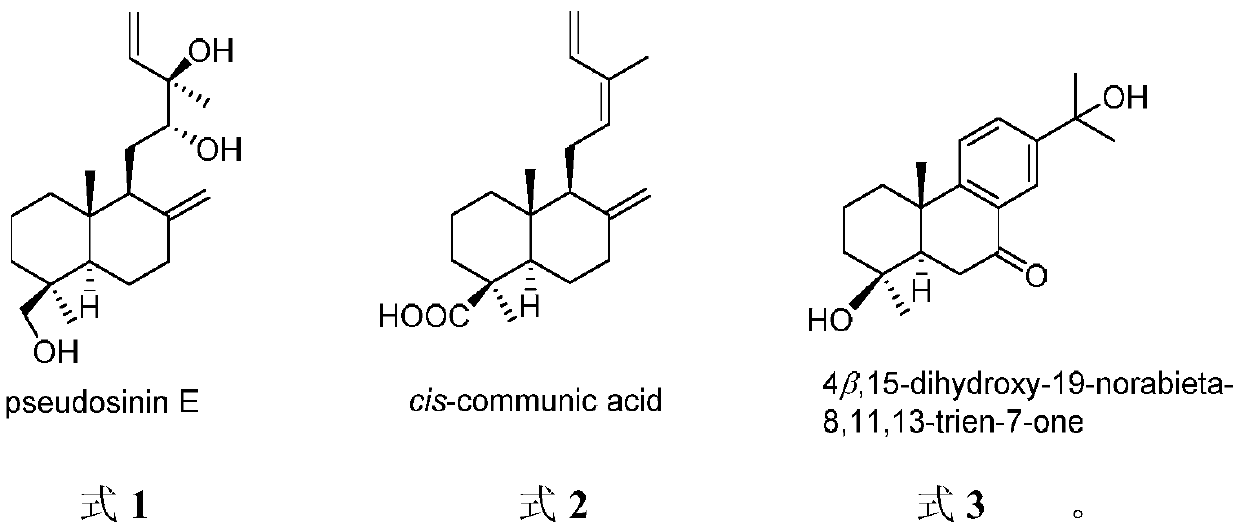
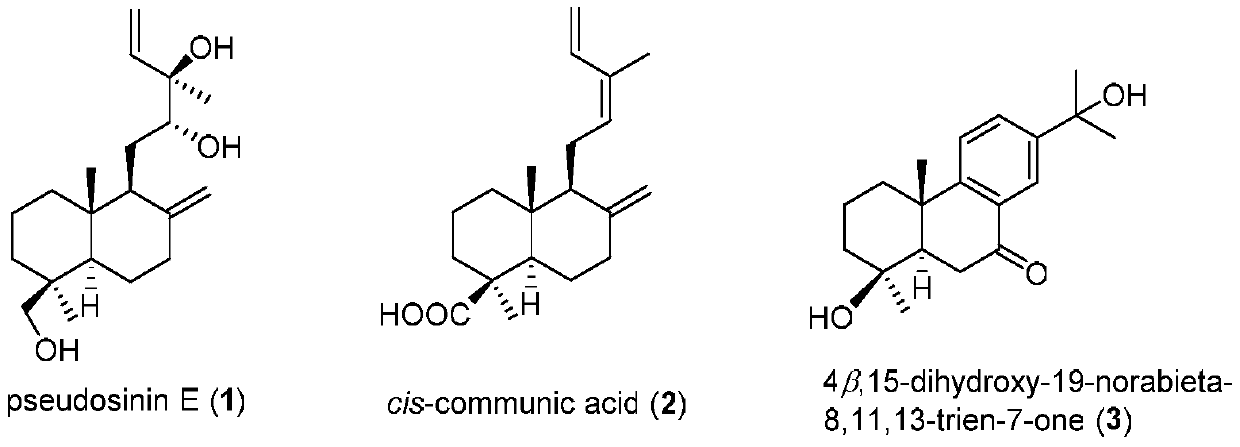
[0023] Take 30kg of Douglas fir pine needles and twigs, crush them and extract them by cold immersion in 90% methanol at room temperature for 5 times, combine the extracts and concentrate under reduced pressure to obtain 3.1kg of extract. The extract was dispersed with water and extracted with petroleum ether, ethyl acetate and n-butanol in sequence, and the ethyl acetate extract was concentrated under reduced pressure to obtain 500 g of extract. The extract was subjected to 100-200 mesh silica gel column chromatography, and was eluted with a gradient of petroleum ether: ethyl acetate 50:1-0:1 and ethyl acetate: methanol 10:1-0:1 to obtain 10 components ( Fr.1-Fr.10). cis-communic acid (25.0 mg) crystallized from subfraction Fr.2. Subcomponent Fr.9 was successively subjected to MCI microporous resin column chromatography (eluted with a gradient of 50%-100% methanol), silica gel column chromatography (20...
Embodiment 2
[0027] Example 2: Determination of ATP-citrate lyase inhibitory activity
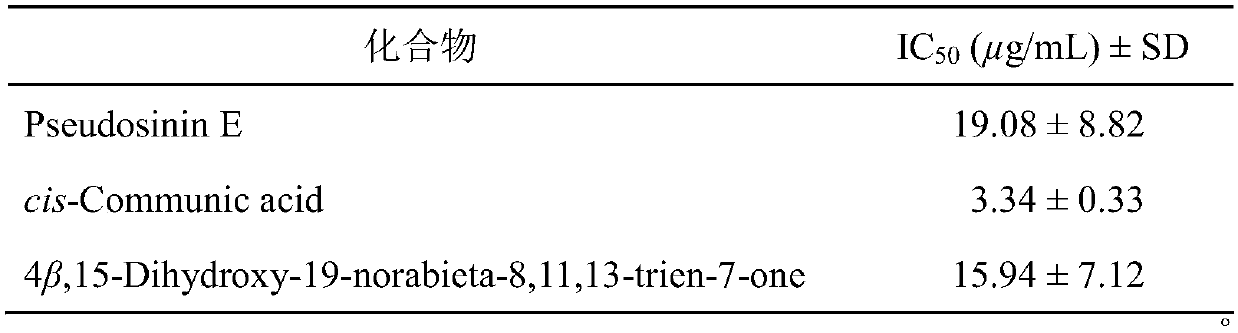
[0028] Experimental method: In this experiment, the ATP-dependent citrate lyase ACL can catalyze the conversion of citric acid into acetyl-CoA, and then produce the precursor molecule of fatty acid synthesis - malonyl-CoA. This reaction is accompanied by the consumption of ATP, so ADP-Glo and kinase detection kits can be used to detect changes in ATP to indirectly reflect the inhibitory effect of compounds on ACL enzyme activity. Specifically, the percent inhibition rate of ACL enzyme activity was investigated when the concentration of the compound was selected as 20 μg / ml in the preliminary screening, and the test results showed that pseudosinin E, cis-communic acid and 4β, 15-dihydroxy-19-norabieta-8 ,11,13-trien-7-one inhibition rates were higher than 61%, 110% and 59%. Further determination of IC 50 Value: The sample is dissolved in DMSO to make a suitable concentration before use, 3-fold diluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com